When metal is placed in soil, it is exposed to a hostile environment that can cause corrosion.
If you do not protect the metal, you will face the problem of destruction, loss of integrity of steel supports and other difficulties.
In this article we will explain in detail what soil corrosion of metal is, how it appears and what factors can aggravate the situation. We will also touch upon issues of protection to minimize the negative impact of an aggressive environment.
Durability of a metal screw pile taking into account corrosion processes in the soil
One of the most significant issues that arise when using metal structures in construction is the issue of the resistance of such structures to corrosion processes and the associated durability of buildings and structures.
Currently, there is a set of interconnected interstate standards that establish general requirements, rules, norms and methods for protecting products, structures and materials from corrosion, aging and biological damage at all stages of the life cycle of products and structures, research and justification for development (ESZKS Standards - Unified System of Protection against corrosion and aging of materials and products) [1, 2, 3, 4].
The purpose of the ESZKS is to ensure and maintain a given level of quality of products, structures and materials using means and methods of protection against corrosion, aging and biodamage, taking into account the requirements of safety, ecology, compatibility and interchangeability, as well as the competitiveness of products and structures on the world market.
In addition to the ESZKS standards, requirements for corrosion resistance are also established by standards for certain types of structures and their parts, depending on the current corrosion factors.
For underground structures (including foundations), the corrosion hazard criteria are:
corrosive aggressiveness of the environment (soils, groundwater and other waters) in relation to the metal of the structure (including biocorrosive aggressiveness of soils);
dangerous effect of stray direct and alternating currents.
Based on these criteria, it follows that the rate of metal corrosion in the soil depends on:
Soil pH. The lower the pH (acidic environment), the higher the corrosion rate.
electrical resistance of soil a. The higher the soil resistance, the slower the corrosion rate.
It is also necessary to take into account the presence of an anti-corrosion coating that prevents corrosion.
Research to determine the electrical resistance of the soil, taking into account a possible increase in humidity and temperature changes, was carried out by the Federal Road Agency and is reflected in the manual for transport engineers (Table 1).
Source
4.2. Underground corrosion
Underground corrosion of metal structures occurs in soil or ground conditions and usually has an electrochemical mechanism.
Mainly metal pipelines, underground tanks, power electrical cables, etc. are susceptible to underground corrosion. Particularly severe destruction is observed under conditions of combined influence of soil and stray currents. The following types of soil corrosion are distinguished:
– Underground corrosion is corrosion in the soil caused by corrosive elements that appear on the metal in places where it comes into contact with a corrosive environment due to the heterogeneity of the metal of the structure, the unequal composition of the soil, differences in temperature, humidity and air conductivity of the soil in different areas of the structure.
– Underground biocorrosion is corrosion caused by the activity of microorganisms acting on metal; usually the process ends with electrochemical corrosion.
– Electrocorrosion – corrosion of metal underground structures under the influence of leakage currents from the rails of electrified railways and other industrial installations. It is divided into corrosion by stray currents and corrosion by external current.
The soil contains moisture and various chemical reagents, so it has ionic conductivity and in most cases, with the exception of very dry soils, the mechanism of underground corrosion is electrochemical. The most characteristic cathodic process in underground conditions is oxygen depolarization. In acidic soils (swampy), corrosion can also occur with hydrogen depolarization. Let's look at an example of how a corrosive element works in soil.
At the anode, the oxidation reaction of iron occurs with the formation of hydrated ions:
The oxygen ionization reaction occurs at the cathode:
Ions in soil electrolyte
And
interact with each other, forming an insoluble precipitate of iron hydroxide, which can then turn into iron oxide:
,
.
Anodic and cathodic processes, in most cases, occur in different areas, i.e. the surface of the corroding metal consists of a certain number of corrosive microelements and the overall corrosion rate depends on the number of such elements and the intensity of their work. This corrosion mechanism is called heterogeneous-electrochemical.
The overall rate of corrosion is determined by the speed of the process that proceeds more slowly than others. The process whose kinetics determines the overall corrosion rate is called controlling.
Depending on the conditions, the following types of control of underground corrosion of metals are possible: predominantly cathodic control - in wet soils; predominantly anodic control - in loose and dry soils; mixed cathode-ohmic control – for ground corrosion of metal structures due to the operation of extended macropairs (pipelines).
In most cases, corrosion of underground structures occurs with predominant cathodic control, due to inhibition of oxygen delivery to the metal surface.
The corrosion hazard criteria for underground metal structures, according to GOST 9.602 - 89, are:
– Corrosive aggressiveness of the environment towards the metal of the structure;
– Dangerous effects of direct and alternating stray currents.
The rate of metal corrosion in soil depends on the corrosive activity of the soil, that is, on some of its properties: structure, porosity, humidity, groundwater salinity, acidity, air conductivity, electrical resistivity and environmental temperature.
– Soil moisture. The presence of moisture makes the soil an electrolyte and causes electrochemical corrosion of metals. An increase in soil moisture accelerates the anodic process, reduces the electrolyte resistance and complicates the cathodic process with a significant water content in the soil. The maximum rate of underground corrosion is observed in soils containing 15-25% moisture. For each soil there is a certain moisture range corresponding to the maximum corrosion rate.
— Air conductivity of soils. Porous soils can retain moisture for a long time and create favorable conditions for aeration (oxygen diffusion). Increasing the air conductivity of soils accelerates the corrosion process due to the facilitation of the cathodic process.
— Electrical conductivity of soils. The presence of water-soluble salts in the soil increases its electrical conductivity. Ions have the greatest influence on the corrosion process.
,
,
,
,
,
,
etc. An increase in soil salinity, in addition, facilitates the occurrence of anodic (depassivation of the anodic areas of the surface) and cathodic processes.
— Soil acidity. It varies widely (pH 3-9). Very acidic soils accelerate the corrosion of metals as a result of increased solubility of secondary corrosion products and possible additional hydrogen depolarization. Based on the pH value, acidic (pH 3-5), neutral (pH 6-8) and alkaline (pH 9-10) soils are distinguished.
— Presence of microorganisms. Microorganisms found in soils can cause significant local acceleration of metal corrosion. The greatest danger is posed by anaerobic sulfate-reducing bacteria, which develop in silt, clay and swampy soils. During their life activity, these bacteria reduce the sulfates contained in the soil, consuming hydrogen generated during the cathodic process, to sulfide ions with the release of oxygen:
The released oxygen takes part in the cathodic depolarization of the corrosion process. The sulfide ion is a depassivator and also binds iron ions, forming low-protective sulfide films, and thereby facilitates the anodic process.
— Soil temperature. Soil temperature affects the kinetics of electrode processes and diffusion, which determine the corrosion rate. Typically, with increasing temperature, an exponential increase in the rate of underground metal corrosion is observed. Temperature differences in certain areas of extended underground structures can lead to the emergence of thermogalvanic corrosive elements that enhance corrosion.
The electrical resistivity of the soil is a function of all the soil properties considered and represents one of the most characteristic indicators of the corrosion activity of soils in relation to steel. Within certain limits, there is a direct relationship: the lower the electrical resistivity, the greater the corrosion rate. This dependence allows us to approximately estimate the corrosion activity of soils.
According to GOST 9.602 - 89, the corrosion activity of soils in relation to steel is assessed:
– according to the electrical resistivity of the soil;
– according to the average cathode current density when the cathode potential is shifted 100 mV negative than the steel corrosion potential.
In table Table 4.2 shows the electrical resistivity of the soil and the average cathode current density, which characterize the corrosion activity of soils towards steel.
Specific electrical resistivity of soil, Ohm∙m
Average cathode current density,
A/m 2
Durability of a metal screw pile taking into account corrosion processes in the soil
Corrosion rate is a multifactorial parameter that depends on both external environmental conditions and the internal properties of the material. In the regulatory and technical documentation there are certain restrictions on the permissible values of metal destruction during the operation of equipment and building structures to ensure their trouble-free operation. In design, there is no universal method for determining corrosion rate. This is due to the complexity of taking into account all factors. The most reliable method is to study the operating history of the facility.
Assessment methods
There are several ways to assess the rate of destruction of metals in aggressive environments:
- Laboratory - testing samples in artificially simulated conditions close to real ones. Their advantage is that they can reduce research time.
- Field – carried out in natural conditions. They take a long time. The advantage of this method is to obtain information about the properties of the metal under further operating conditions.
- Full-scale – testing of finished metal objects in a natural environment.
Source
Criteria
You might be interested in:Isobaric, isochoric, isothermal and adiabatic processes for an ideal gas
Currently, several corrosion rate indicators are used in equipment design:
- According to the direct method of assessment: reduction in the mass of a metal part per unit surface - weight indicator (measured in grams per 1 m2 per 1 hour); depth of damage (or permeability of the corrosion process), mm/year; the amount of released gas phase of corrosion products; the length of time during which the first corrosion damage appears; the number of corrosion centers per unit surface area that appeared over a certain period of time.
- According to indirect assessment: current strength of electrochemical corrosion; electrical resistance; change in physical and mechanical characteristics.
Channel PROGRAMMER'S DIARY
The life of a programmer and interesting reviews of everything. Subscribe so you don't miss new videos.
The first indicator using the direct assessment method is the most common.
Depth corrosion index
8.76 is the coefficient for the transition from measuring the weight indicator of the corrosion rate per 1 hour to the depth indicator per 1 year (24 hours • 360 = 8760 hours);
v—corrosion rate, g/(m 2 •h);
If corrosion is local in nature, its speed cannot be accurately characterized by weight or depth. For pitting corrosion, it is necessary to determine the maximum depth index. With intergranular corrosion and corrosion cracking, the corrosion rate is quantitatively characterized by a mechanical corrosion indicator , for example, by loss of strength:
σ —tensile strength before corrosion;
σ1 is the ultimate strength after corrosion, calculated in relation to the initial cross-sectional area of the metal sample;
Source
Calculation formulas
In the general case, weight losses that determine the rate of metal corrosion are found using the following formula:
where q is the reduction in metal mass, g;
S – surface area from which the material was transferred, m2;
For sheet metal and shells made from it, the depth indicator is determined (mm/year):
m is the depth of penetration of corrosion into the metal.
There is the following relationship between the first and second indicators described above:
where ρ is the density of the material.
The influence of various factors on soil corrosion of metals
The ground conditions in which metal structures are operated are very different. The rate of corrosion of metals in soil largely depends on the composition of the soil, its moisture capacity (i.e., the ability to retain moisture) and air permeability and is determined by the kinetics of electrode processes, and in the case of the operation of extended corrosion pairs, also by the ohmic resistance of the soil. The following main factors that determine the rate and nature of soil corrosion of metals should be noted:
Reducing the rate of metal corrosion
The damage caused by corrosion is not limited to the destruction of the products or metal parts themselves. In addition to the fact that under its influence already manufactured items become unusable, the efforts and labor of people spent on production are lost. The main reason for the costs is the replacement or repair of parts that fail under the influence of this process.
Depending on where and how the products are used, and on the presence of the metal in the ground, in the air, during the creation of underwater pipelines or ships, two types of impact of this process are distinguished:
- Chemical. Corrosion, called “chemical”, is observed in dry gases and substances that do not conduct electricity. It occurs in blast furnaces, during rolling or forging of steel. Substances in this process include carbon disulphide, kerosene, and gasoline. Chemical corrosion can be observed in car engines and their gasoline tanks, petrochemical equipment, and oil pipelines.
- Electrochemical. Electrochemical corrosion is accompanied by the formation of low-voltage electric currents and proceeds according to the principle of galvanics, when the metal and the environment (sea, river water, damp soil, humid atmosphere, acids, bases) serve as a cathode and anode.
In the case of uniform corrosion, the rate can be determined by the formula:
v is the corrosion rate, which is usually expressed in the following units: g/(m2•h) or mg/(cm2•day);
Δm—loss (increase) of mass;
Reducing the rate and depth of corrosion is the main goal of protecting iron and its alloys from destruction caused by this process. Reducing rust damage to metal parts and structures is achieved in several ways:
- changes in environmental factors acting on the metal;
- by producing anti-corrosion alloys;
- applying a layer of coating that is not subject to corrosion;
- spraying on the surface of the product metals that are more resistant to the environment that causes this phenomenon;
- protection is carried out using electrochemical methods.
Changing the environment that causes rust is achieved by introducing various corrosion inhibitors into it. This method is increasingly used to reduce steel corrosion.
Steel is the most common type of metal alloy used by humans, which is produced by smelting and mixing with various elements that create the necessary qualities of the resulting material. Due to this, steel corrosion can be reduced.
Chemical elements are added at the production stage, and these additives do not affect the overall performance of the metal. Alloyed and stainless steels are produced using this method.
Coatings that prevent rusting or slow it down are called anti-corrosion.
Layers can be applied by paint and varnish and galvanic methods. Sometimes they are combined to obtain a coating in which steel corrosion is reduced to a minimum, which expands the range of application of the material.
Electrochemical corrosion protection is one that directly affects the change in the potential of an iron part depending on the area of use. This reaction is carried out when the place of use of the product is known. It can be anodic or cathodic.
The most unpleasant thing about this phenomenon is that rusting (corrosion of steel) causes the destruction or reduction in strength of finished products that directly affect human life.
For example, accidents on various pipelines supplying gas and oil; breakdowns or collapse of drawbridges, metal structures, cranes.
Main factors influencing the corrosion rate
The rate of metal destruction is influenced by the following groups of factors:
- internal, related to the physical and chemical nature of the material (phase structure, chemical composition, surface roughness of the part, residual and operating stresses in the material, and others);
- external (environmental conditions, speed of movement of a corrosive environment, temperature, atmospheric composition, presence of inhibitors or stimulants, and others);
- mechanical (development of corrosion cracks, destruction of metal under the influence of cyclic loads, cavitation and fretting corrosion);
- design features (choice of metal grade, presence of gaps between parts, roughness requirements).
DETERMINING CORROSION RATE FROM ACTUAL WALL THICKNESS MEASUREMENTS
2.1. The results of periodic measurements of the wall thickness of a vessel or pipeline serve as the basis for determining the rate of metal corrosion under operating conditions.
2.2. Wall thickness measurements are made using non-destructive testing methods or by drilling and measuring the wall thickness with a measuring tool. Preference should be given to ultrasonic thickness gauging.
2.3. If the results of wall thickness measurements using non-destructive testing methods are in doubt, then the measurement should be made by through drilling.
2.4. On vessels and pipelines operating in environments that cause intergranular corrosion or stress corrosion cracking, through drilling, followed by their sealing using arc welding methods, is not allowed.
2.5. The place and method of measuring the wall thickness of a vessel or pipeline is determined based on the results of their technical examination by technical supervision services, taking into account the characteristics of corrosion lesions in various parts of vessels and pipelines.
2.6. The locations of the measurement points, the measurement method and the measurement results must be documented in a corrosion map for the vessel or pipeline and stored in the passport (see maps SZK-2 and SZK-3).
Source
Physicochemical characteristics
You may be interested in: Such ordinary people, or the meaning of “why not”
The most important among internal corrosion factors are the following:
- Thermodynamic stability. To determine it in aqueous solutions, Pourbaix reference diagrams are used, the abscissa of which is the pH of the medium, and the ordinate is the redox potential. A positive shift in potential means greater stability of the material. It is roughly defined as the normal equilibrium potential of the metal. In reality, materials corrode at different rates.
- Atom position in the periodic table of chemical elements. The metals most susceptible to corrosion are alkali and alkaline earth metals. The rate of corrosion decreases as the atomic number increases.
- Crystal structure. It has an ambiguous effect on destruction. The coarse-grained structure itself does not lead to an increase in corrosion, but is favorable for the development of intercrystalline selective destruction of grain boundaries. Metals and alloys with a uniform phase distribution corrode uniformly, while those with a non-uniform phase distribution corrode according to a focal mechanism. The mutual arrangement of the phases performs the function of anode and cathode in an aggressive environment.
- Energy heterogeneity of atoms in a crystal lattice. Atoms with the highest energy are located in the corners of the edges of microroughness and are active centers of dissolution during chemical corrosion. Therefore, careful mechanical processing of metal parts (grinding, polishing, finishing) increases corrosion resistance. This effect is also explained by the formation of denser and more continuous oxide films on smooth surfaces.
Influence of acidity of the environment
You might be interested in: Comic nominations for teachers for graduation
During the process of chemical corrosion, the concentration of hydrogen ions affects the following points:
- solubility of corrosion products;
- formation of protective oxide films;
- metal destruction rate.
At a pH in the range of 4-10 units (acidic solution), corrosion of iron depends on the intensity of oxygen penetration to the surface of the object. In alkaline solutions, the corrosion rate first decreases due to surface passivation, and then, at pH>13, increases as a result of the dissolution of the protective oxide film.
Each type of metal has its own dependence of the intensity of destruction on the acidity of the solution. Noble metals (Pt, Ag, Au) are resistant to corrosion in acidic environments. Zn and Al are quickly destroyed in both acids and alkalis. Ni and Cd are resistant to alkalis, but easily corrode in acids.
Results of metal corrosion. Methods for determining corrosion rate.
Whatever type of corrosion occurs, the metal is destroyed as a result. First of all, the appearance of the product suffers: the smooth polished surface becomes dull, then rough and, finally, covered with various chemical compounds - corrosion products. It penetrates deep into metal products, completely destroying them.
Method for determining metal weight loss. Corrosion losses.
To determine the corrosion resistance values of different metals, several rating scales are used. The most common assessment method is based on metal weight loss.
The method involves weighing the part before it corrodes, and then, after removing the corrosion from the surface of the part, the metal loss is calculated from the difference in weight.
It is customary, for example, to consider a metal to be quite stable if the loss in weight of the substance is no more than 0.1 g per 1 m2/h of surface. When the loss of metal is from 3 to 10 g m2/h, the metal is considered low-resistant; with a greater loss, it is considered unstable.
Determination of metal corrosion rate.
Sometimes corrosion resistance is determined by the amount of hydrogen released, for example, when a metal is dissolved in acids.
A metal sample, pre-treated accordingly, is immersed in an electrolyte solution, for example an acid, and covered with a funnel with a burette on it. The burette is filled with the same acid solution. The released gas (usually hydrogen) enters the burette through the funnel and displaces the acid solution from it.
The corrosion resistance of the metal is judged by the amount of hydrogen released, as well as by the rate of its release.
This method is often used to determine the rate of chemical interaction of a metal with acids such as sulfuric and hydrochloric, and also sometimes to determine the dissolution characteristics of aluminum and zinc in solutions of alkalis and acids.
Results of metal corrosion.
However, methods for assessing corrosion damage by weight loss or hydrogen release do not always provide an idea of both the nature of the corrosion process itself and its possible consequences.
Scale for rough assessment of corrosion resistance of metals
| Durability group | Depth corrosion rate, mm/year | Point |
| Absolutely resistant | 0,001 | 1 |
| Very resistant | 0,001 … 0,005 | 2 |
| 0,005 … 0,010 | 3 | |
| Persistent | 0,01 … 0,05 | 4 |
| 0,05 … 0,10 | 5 | |
| Low resistant | 0,1 … 0,5 | 6 |
| 0,5 … 1,0 | 7 | |
| Low-resistant | 1,0 … 5,0 | 8 |
| 5,0 … 10,0 | 9 | |
| Unstable | 10,0 | 10 |
Composition and concentration of neutral solutions
The rate of corrosion in neutral solutions depends to a large extent on the properties of the salt and its concentration:
- The hydrolysis of salts in a corrosive environment produces ions that act as activators or retarders (inhibitors) of metal destruction.
- Those compounds that increase pH also increase the rate of the destructive process (for example, soda ash), and those that reduce acidity reduce it (ammonium chloride).
- In the presence of chlorides and sulfates in the solution, destruction is activated until a certain salt concentration is reached (which is explained by the intensification of the anodic process under the influence of chlorine and sulfur ions), and then gradually decreases due to a decrease in oxygen solubility.
Some types of salts are capable of forming a poorly soluble film (for example, iron phosphate). This helps protect the metal from further destruction. This property is used when using rust neutralizers.
Classification of types of corrosion and methods of protection against it
Corrosion is a dangerous process of degradation (i.e. destruction) of the top layer of metals under the influence of various environments. Corrosion is usually caused by the chemical environment that surrounds the metal.
Regardless of the type of structure and its operation, the simplest and most understandable method for combating this phenomenon is to “protect against corrosion” using protective paints and special varnishes.
Main types of corrosion
Speaking about the mechanism of the corrosion process, we can notice 2 main types of corrosion: chemical and electrochemical.
Chemical is a clear result of the passage of reactions during which, after the destruction of the metal bond, parts of the metal and all the atoms that are included in the oxidizing agents create a strong bond.
An electric current cannot occur between different parts of a metal surface. This type of corrosion may be inherent in chemical media that are not able to transmit electric current.
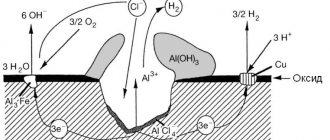
Corrosion of screw piles. How to extend the service life of a pile-screw foundation?
For foundations on screw piles, the greatest danger is posed by two subtypes of electrochemical corrosion - soil and atmospheric.
Soil corrosion is the destruction of underground metal structures under the influence of soil electrolyte. On the surface of metal products in contact with soil electrolyte, due to local inhomogeneities of the metal or electrolyte, a large number of corrosive elements appear.
However, we must not forget that soils and soils are extremely diverse, not only within large regions, but also within one small area. That is, in a relatively small area there can be soils with varying degrees of corrosive aggressiveness: highly corrosive (heavy clayey soils that retain moisture for a long time), moderately corrosive (loams) and practically inert in terms of corrosion (sandy loam, sandy soils).
The difference in the course of corrosion processes in different soils is also indicated by the British Standard BS 8004 “Foundations” (clause 10.3.5). In accordance with this document, the residual thickness of steel piles installed in undisturbed soils “remains within acceptable thickness values even after many decades of operation,” since the corrosion rate in these soils does not exceed 1-2 mm per 100 years. However, in disturbed soils, “the use of redox potential, soil resistivity, and pH values may have some value in predicting corrosion rates.” However, even in this case, the thickness of the metal should be selected based on the degree of aggressiveness of the disturbed soils.
Corrosion inhibitors
Corrosion retarders (or inhibitors) differ in their mechanism of action on the redox process:
- Anode. Thanks to them, a passive film is formed. This group includes compounds based on chromates and dichromates, nitrates and nitrites. The last type of inhibitors is used for inter-operational protection of parts. When using anodic corrosion inhibitors, it is necessary to first determine their minimum protective concentration, since addition in small quantities can lead to an increase in the rate of destruction.
- Cathode. The mechanism of their action is based on reducing the oxygen concentration and, accordingly, slowing down the cathodic process.
- Shielding. These inhibitors isolate the metal surface by forming insoluble compounds that are deposited as a protective layer.
PURPOSE AND GENERAL PROVISIONS
1.1. This instruction is intended to determine the actual rate of metal corrosion of the walls of vessels and pipelines operated at enterprises of the USSR Ministry of Petroleum and Chemical Industry, in order to establish the frequency of their technical examination in accordance with the requirements of current rules and regulations.
1.3. If it is impossible or difficult to apply the methods set out in clause 1.2, the corrosion rate is determined approximately using witness samples or by assessing the corrosivity of the environment in relation to a given metal using corrosion probes.
1.4. The corrosion rate is determined for each vessel and pipeline of a process unit, line, or workshop. For a group of vessels or pipelines operating in a given technological installation, line, workshop in the same environment under the same operating conditions and material design, the corrosion rate is determined using a selected representative object.
1.5. The rate of metal corrosion of the walls of vessels and pipelines is subject to clarification in each case of a significant change in their operating conditions (working environment, temperature, pressure), affecting the corrosiveness of the working environment, or in the case of replacement of material design.
1.6. At each enterprise that owns the vessels, a list of vessels is compiled and approved by the chief engineer, indicating the corrosion rate of the body metal. Information on the corrosion rate of pipelines is entered into the pipeline passport.
When special types of corrosion damage such as corrosion cracking, intergranular corrosion or delamination along the wall thickness are detected, information about this is also entered into the passport of the vessel or pipeline, and issues of further operation or repair of vessels and pipelines with such damage must be agreed upon with a specialized organization.
Methods to reduce corrosion: mechanism and effectiveness
The ability of a painted surface to resist corrosion processes depends on which corrosion mechanism predominates. For example, with constant exposure to a chemically active medium over time, the potential difference between the outer surface of a metal product and its internal volumes changes significantly. In this case, corrosive currents arise, intensifying the corrosion process (a phenomenon that often causes the destruction of steel pipes in underground pipelines). Here, painting does not have any effect, since the chemical composition of the surface covered with a layer of paint does not change over time.
Metal coating
It’s a different matter when the surface is covered with a metal that has a negative electrolytic potential with respect to redox processes. When oxidation reactions predominate, it is more effective to protect steel by applying a surface coating containing aluminum and zinc - metals that, in terms of their oxygen activity, are “to the left” of iron.
Such processes - galvanizing and aluminizing - are widely used in the practice of anti-corrosion protection of steel components and individual parts located in an oxidizing environment. Painting in these situations is of an auxiliary nature, to increase the decorative characteristics of the surface.
In a reducing environment, the process of formation of iron hydrides can be effectively blocked by creating surface coatings of metals located “to the right” of hydrogen: these are copper and all noble metals. Copper plating, although used in practice, is usually performed for relatively small surfaces, since it is a very costly process in terms of finances. It is for such situations that coloring can and should be used.
Coloring
The protective role of paints is that they always contain corrosion inhibitors - components that slow down the rate of scaling processes over time. The chemical formulas of inhibitor substances are designed in such a way that, as a result, the appearance of rust is stopped. The elasticity of modern coloring compositions allows coatings to successfully withstand surface stresses, which provoke the onset of corrosion processes.
The anti-corrosion properties of paints increase if they contain organosilicon polymers, which increase the ability of the painted surface to withstand changes in humidity and temperature, regardless of the time of year. However, such paints have two significant disadvantages:
- poisonous;
- are ineffective under conditions of the electrolytic corrosion mechanism.
Thus, properly selected coloring compositions can effectively block corrosion processes. To do this, they must contain corrosion inhibitors, have sufficient elasticity and mechanical strength that changes slightly over time.
Mechanical impact
Increased corrosion in an aggressive environment is facilitated by such types of mechanical impact as:
- Internal (during molding or heat treatment) and external (under the influence of externally applied load) stresses. As a result, electrochemical heterogeneity occurs, the thermodynamic stability of the material decreases, and corrosion cracking occurs. Destruction occurs especially quickly under tensile loads (cracks form in perpendicular planes) in the presence of oxidizing anions, for example, NaCl. A typical example of devices susceptible to this type of destruction are parts of steam boilers.
- Alternating dynamic effects, vibrations (corrosion fatigue). There is an intensive decrease in the fatigue limit, multiple microcracks are formed, which then merge into one large one. The number of cycles before failure largely depends on the chemical and phase composition of metals and alloys. Pump axles, springs, turbine blades and other equipment elements are susceptible to such corrosion.
- Friction of parts. Rapid corrosion is caused by mechanical wear of protective films on the surface of the part and chemical interaction with the environment. In liquid, the rate of destruction is lower than in air.
- Cavitation impact. Cavitation occurs when the continuity of fluid flow is disrupted as a result of the formation of vacuum bubbles, which collapse and create a pulsating effect. As a result, deep local damage occurs. This type of corrosion is often observed in chemical apparatus.
Practice of corrosion testing of metals
Indicators of corrosion are climatic factors - temperature, composition and relative humidity of the environment, the nature of the distribution of external loads. It is also necessary to take into account changes in illumination by time of day, amount of precipitation, and possible air pollution. For example, in areas of smoke waste emissions near chemical plants and metallurgical plants, accompanied by a sharp increase in the percentage of SO2, corrosion processes are sharply intensified.
Quantitative dependences of corrosion on time can be used as indicators of corrosion activity:
- Linear - most often this is typical for metal surfaces that do not have a protective coating.
- Exponentially decreasing - found during acid corrosion of ordinary metals and alloys.
- Exponentially increasing - when there is a protective coating on the surface of the part.
The intensity of rust formation under such conditions is reduced by:
- low wind speed;
- reduced cyclicity in time of changes in relative humidity indicators;
- the nature of the impact of a corrosive environment on the surface.
With little or no wind, there are no conditions for mixing the flow washing the contact surface of the steel. During long phases of low and high humidity throughout the year, a film of surface rust has time to form, swell and separate from the base metal. The thickness of the surface will decrease, but the corrosion processes are forced to “start” all over again, and this requires not only time, but also suitable conditions - wind or changes in the chemical composition of the air, which is not always the case.
Moisture, acid or alkali can reach the surface of the steel in the form of drops or streams. The first method is typical for areas with high precipitation, and the second is for the unfavorable environment in which the part or metal structure operates.
Design factors
You might be interested in: Dig or drip? How to write correctly?
When designing elements operating in aggressive conditions, it must be taken into account that the corrosion rate increases in the following cases:
- upon contact of dissimilar metals (the greater the difference in electrode potential between them, the higher the current strength of the electrochemical destruction process);
- in the presence of mechanical stress concentrators (grooves, grooves, holes, etc.);
- with low cleanliness of the treated surface, since in this case local short-circuited galvanic pairs arise;
- when there is a significant difference in the temperature of individual parts of the apparatus (thermogalvanic elements are formed);
- in the presence of stagnant zones (cracks, gaps);
- during the formation of residual stresses, especially in welded joints (to eliminate them, it is necessary to provide heat treatment - annealing).