Main varieties
During corrosion in electrolytes, chemical energy is converted into electrical energy. In this regard, it is called electrochemical. It is customary to distinguish the following types of electrochemical corrosion.
Intercrystalline
Intergranular corrosion refers to a dangerous phenomenon in which the grain boundaries of nickel, aluminum and other metals are destroyed in a selective manner. As a result, the strength and plastic properties of the material are lost. The main danger of this type of corrosion is that it is not always visually noticeable.
Pitting
Pitting electrochemical corrosion is a point lesion of individual areas of the surface of copper and other metals. Depending on the nature of the lesion, closed, open, and superficial pitting are distinguished. The size of the affected areas can vary from 0.1 mm to 1.5 mm.
Slotted
Crevice electrochemical corrosion is commonly called the intensified process of destruction of metal structures in the locations of cracks, gaps and cracks. Crevice corrosion can occur in air, gas mixtures, and sea water. This type of destruction is typical for gas pipelines, the bottoms of sea vessels and many other objects.
Corrosion occurs in conditions of a small amount of oxidizer due to the difficult approach to the crack walls. This leads to the accumulation of corrosive products inside the gaps. The electrolyte contained in the internal space of the gap can change under the influence of hydrolysis of corrosion products.
In order to protect metals from crevice corrosion, it is common to use several methods:
- sealing gaps and cracks;
- electrochemical protection;
- inhibition process.
As preventive methods, you should use only those materials that are least susceptible to rust, and also initially correctly and rationally design gas pipelines and other important objects.
Competent prevention in many cases is a simpler process than subsequent cleaning of metal structures from ingrained rust.
Rules for gutting and cutting pike
The pike is gutted and cut after cleaning the scales.
How to properly cut pike into cutlets:
The belly of the fish is cut open and the entrails are pulled out.
It is important not to damage the bile, otherwise the product may be spoiled. The esophagus is removed along with the gills, leaving the head
The white film running along the belly (also known as the swim bladder) is removed. The head is cut off with a knife.
After that, they move on to cutting up the carcass itself. This procedure will vary depending on what you plan to cook: minced, filleted, whole pieces or stuffed fish.
For minced meat
If you plan to prepare minced meat for cutlets, only large bones (spine) are removed. The vertebral column of the pike is strong and hard. If the pike is small, then the spine does not need to be removed.
Cutting medium and large pike for minced meat involves the following steps:
- The fish is placed on a cutting board.
- The knife blade is placed parallel to the spine (in the place where the head was) and the fillet is cut to the tail. The knife should be held as close to the ridge as possible.
- In the same way, cut the fillet from the other side of the fish.
- The middle part with the spine is removed; it will be useful for preparing fish soup.
- The pike for cutlets is deboned, but not thoroughly, because... During the process of grinding meat into minced meat, the bones crumble and are not felt in the finished cutlets. The fillet is scrolled in a meat grinder through a fine grill. To better grind the seeds, this can be done twice.
For frying fillets
There are many bones in the pike's body, among them there are massive (spine), medium and very thin. There are a lot of seeds and it is difficult to remove them.
The boneless product is prepared in the following order:
- The fillet is cut along the entire length of the carcass.
- The pike is turned over and the fillet is cut off on the other side.
- The remaining ridge and tail section are cut into pieces and set aside.
- The ribs are cut from the fillet. They are very thin; if the knife is sharp, there will be no significant loss of product.
- Fins are cut out.
- The flesh with the bones is felt above the rib line and two parallel cuts are made along the fillet.
- The strip with bones is separated with a knife and cut out of the fillet.
- The remaining bones are removed with tweezers. A layer of boneless fish pulp is obtained.
- The skin is removed from the fish.
- The fillet is cut into portions and the dish is prepared according to the chosen recipe.
For frying in pieces
Preparation for frying in pieces does not take much time.
The carcass is processed as follows.
- The fins are cut off from the pike.
- The tail is cut off.
- Cut the fish into pieces 1.5-2 cm thick. If you plan to bake the pike in the oven, the length of the pieces can reach 10-15 cm.
- If the pike is large, the pieces are cut randomly to make it convenient to place them in the pan.
For stuffing
Stuffed pike (with and without head) is prepared in the oven. Medium-sized fish are suitable for this dish.
Cutting steps:
- An incision is made around the pike head.
- The skin along with the fins is removed from the carcass using a knife and scissors. To ensure that the skin is easily removed, the carcass is beaten with a rolling pin.
- The pike meat is cut off (and picked by hand) from the remaining part of the carcass. All that remains is the skeleton of the fish.
- Prepare minced meat for filling using fillet, vegetables, herbs, meat, eggs, and other ingredients.
- The removed skin is stuffed with minced meat, and the abdomen is sewn up. The dish is prepared in the oven.
How different types of corrosion manifest themselves
An example of the corrosion process is the destruction of various devices, car components, as well as any structures made of metal and located:
- in atmospheric air;
- in waters - seas, rivers contained in the soil and under layers of soil;
- in technical environments, etc.
During the rusting process, the metal becomes a multi-electron galvanic cell. So, for example, if copper and iron come into contact in an electrolytic medium, copper is the cathode and iron is the anode. By donating electrons to copper, iron in the form of ions enters the solution. Hydrogen ions begin to move towards copper and are discharged there. Becoming more and more negative, the cathode soon becomes equal to the potential of the anode, as a result of which the corrosion process begins to slow down.
Different types of corrosion manifest themselves in different ways. Electrochemical corrosion manifests itself more intensely in cases where the cathode contains inclusions of metal with less activity compared to the corroding one - rust appears on them faster and is quite expressive.
Atmospheric corrosion occurs in humid air and normal temperatures. In this case, a film of moisture with dissolved oxygen forms on the surface of the metal. The process of metal destruction becomes more intense as air humidity and the content of gaseous oxides of carbon and sulfur increase, provided that:
- cracks;
- roughness;
- other factors that facilitate the condensation process.
Soil corrosion most affects a variety of underground structures, gas pipelines, cables and other structures. The destruction of copper and other metals occurs due to their close contact with soil moisture, which also contains dissolved oxygen. Destruction of pipelines can occur as early as six months after their construction if the soil in which they are installed is characterized by high acidity.
Under the influence of stray currents emanating from foreign objects, electrical corrosion occurs. Its main sources are electric railways, power lines, as well as special installations operating on direct electric current. To a greater extent, this type of corrosion provokes destruction:
- gas pipelines;
- all kinds of structures (bridges, hangars);
- electrical cables;
- oil pipelines.
The action of the current provokes the emergence of electron entry and exit areas - that is, cathodes and anodes. The most intense destructive process is in areas with anodes, so rust is more noticeable there.
Corrosion of individual components of gas and water pipelines can be caused by the fact that the installation process is mixed, that is, it occurs using different materials. The most common examples are pitting corrosion that occurs in copper elements, as well as corrosion of bimetals.
With a mixed installation of iron elements with copper and zinc alloys, the corrosion process is less critical than with copper casting, that is, with alloys of copper, zinc and tin. Pipeline corrosion can be prevented using special methods.
Causes of corrosion
To protect your car from rust, it is worth understanding the principle of this process. In simple words, corrosion is the formation of rust. To understand the reasons, it’s worth remembering physics from school.
Each conductor acts as an electron transmitter. If you imagine a conductor visually, then it is some kind of metal body surrounded by a cloud of numerous electrons leaving the “shelter” under the influence of heat energy. In the absence of interference, these same electrons come back to the conductor. If a metal element is dipped into an electrolyte, then the metal atoms (with the “+” sign) are transferred to a new composition. The result of the action is the acquisition by the metal of a potential that can be measured.
Corrosion in electrolytic liquid is especially active if the conductor has less activity. The metal element with greater activity acts as the anode, and the less active acts as the cathode. During the interaction, the anode corrodes. The appearance of rust (corrosion) occurs through the following reactions - reduction and oxidation. In this case, the cathode is restored, and the anode is destroyed (covered with rust).
If you place a metal in an aqueous environment or provide contact with a conductor that has less activity, then a corrosion process occurs. The situation gets worse if there is salt in the water. The latter makes the electrolyte conductive, and this leads to an even greater rate of oxidation. Compared to a car and road conditions, in winter transport faces the problems described above. The metal comes into contact with water and a special composition that coats the roads. Acid rain, which has become common in many regions of the country, is also dangerous for metal.
The main indicator is the rate of rust coverage. There is a special parameter here that allows you to determine the resistance of a particular metal to corrosion. Classic iron is characterized by a corrosion rate of 0.03-0.05 mm per year. This means that after five years of operation the metal becomes 0.15-0.25 mm thinner. If no action is taken, a hole may form on the body, which will cost a lot of money to fix.
From what was discussed above, the conclusion suggests itself that to protect a metal from corrosion, it is enough to transform it from an anode to a cathode. Car enthusiasts often use a simple option - they cover the body with special protection. But the latter is effective only on an intact body. The appearance of a crack or scratch on the paintwork leads to contact of the metal with a less active conductor. The result is the appearance of corrosion. Cathodic protection is more effective because it changes the role of the car body, turning it from an anode susceptible to destruction into a resistant cathode.
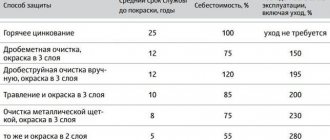
Methods of rust protection
Various methods are used to combat insidious rust. Let's look at those that are the most effective.
Method No. 1
One of the most popular methods is electrochemical protection of cast iron, steel, titanium, copper and other metals. What is it based on?
Electrochemical processing of metals is a special method aimed at changing the shape, size and roughness of the surface by anodic dissolution in an electrolyte under the influence of electric current.
To ensure reliable protection against rust, it is necessary to treat metal products with special means, which contain various components of organic and inorganic origin, even before using them. This method allows you to prevent rust from appearing for a certain time, but later you will have to renew the coating.
Scheme of cathodic protection of pipelines
Electrical protection is a process in which a metal structure is connected to an external source of direct electrical current. As a result of this, polarization of cathode-type electrodes is formed on its surface, and all anode regions begin to transform into cathode ones.
Electrochemical processing of metals can occur with the participation of an anode or cathode. In some cases, alternating processing of a metal product with both electrodes occurs.
Cathodic corrosion protection is necessary in situations where the metal to be protected does not have a predisposition to passivation. An external current source is connected to the metal product - a special cathodic protection station. This method is suitable for protecting gas pipelines, as well as water supply and heating pipelines. However, this method has certain disadvantages in the form of cracking and destruction of protective coatings - this occurs in cases of a significant shift in the potential of the object in the negative direction.
Method No. 2
Electrospark processing of metals can be carried out using various types of installations - non-contact, contact, and also anodic-mechanical.
Method No. 3
To reliably protect gas pipelines and other pipelines from rust, a method such as electric arc spraying is often used. The advantages of this method are obvious:
- significant thickness of the protective layer;
- high level of performance and reliability;
- use of relatively inexpensive equipment;
- simple technological process;
- possibility of using automated lines;
- low energy costs.
Among the disadvantages of this method are low efficiency when processing structures in corrosive environments, as well as insufficient adhesion strength to the steel base in some cases. In any other situations, such electrical protection is very effective.
Method No. 4
To protect a variety of metal structures - gas pipelines, bridge structures, all kinds of pipelines - effective anti-corrosion treatment is required.
This procedure is carried out in several stages:
- thorough removal of fatty deposits and oils using effective solvents;
- cleaning the treated surface from salts soluble in water is carried out using professional high-pressure apparatus;
- removal of existing structural errors, alignment of edges - this is necessary to prevent chipping of the applied paint coating;
- thorough cleaning of the surface using a sandblaster - this is done not only to remove rust, but also to give the desired degree of roughness;
- application of anti-corrosion material and an additional protective layer.
Correct pre-treatment of gas pipelines and all kinds of metal structures will provide them with reliable protection from electrochemical corrosion during operation.