Causes
Causes of corrosion processes:
- contact of different types of metals, alloys;
- frequent temperature changes;
- friction between metal surfaces;
- prolonged exposure to moisture;
- influence of acids, alkalis, chemical elements;
- use of low-quality liquids during mechanical processing of materials;
- grease stains remaining on metal surfaces after touching them.
Rust can form from periodic exposure to static or direct current.
Which type of corrosion destruction is the most dangerous?
It is impossible to give a definite answer.
It all depends on the operating conditions of the products. For example, intergranular corrosion and stress-corrosion cracking are the most dangerous for structural structural elements. For tanks and pipelines, pitting or pitting corrosion is the most dangerous.
For friction surfaces, electrical contacts, and high-frequency waveguides, continuous corrosion is the most dangerous.
In some cases, it is not so much the corrosion itself that is dangerous, but the contamination of the surface with its products (decorative coatings, mirrors, etc.). In this case, the most undesirable is continuous corrosion of a uniform type.
Kinds
Corrosion processes are classified depending on different criteria. The main ones are color, mechanism of rust formation, type of aggressive environment, nature of destruction.
By color
Depending on the color, there are different types of rust. It can be black, yellow, brown, red. The shade depends on the chemical formula of the resulting substance.
Rusty metal
Yellow
The chemical formula of yellow rust is FeO(OH)H2O. It appears under the influence of high humidity, in an environment with a small amount of oxygen. This type of rust can be seen underwater.
Brown
The chemical formula of brown rust is Fe2O3. It is extremely rare and appears without exposure to moisture.
Red
The chemical formula of red rust is Fe2O3•H2O. Formed by simultaneous exposure to water and oxygen. It is more common than other species. The destructive process proceeds evenly, gradually spreading over the entire surface.
Black
Chemical formula: Fe3O4. Appears without exposure to moisture, in an environment with a small amount of oxygen. Often used to create superconductors because it is ferromagnetic.
According to the flow mechanism
Kinds:
- chemical;
- electromechanical.
The processes differ in the mechanism of material destruction.
Chemical
The process of metal destruction, provoking the disintegration of metal bonds, the development of chemical reactions between the atoms of the material. Elements that interact with each other are not spatially separated. The rate of destruction of a part depends on the speed of the chemical reaction.
Electrochemical
This process of destruction of metal parts occurs in an electrolyte environment and is combined with the generation of current.
Rusty ship
By type of aggressive environment
Kinds:
- Atmospheric.
- Gas.
- Radiation.
- Underground.
- Contact.
- Biocorrosion.
- Electrical corrosion.
- Corrosive cavitation.
- Stress corrosion.
- Fretting corrosion.
Atmospheric
Natural process of destruction. Can occur in air or gas atmosphere. An important condition is a high level of humidity. The higher it is, the faster the material will collapse.
Gas
The process of destruction of metal parts, which occurs in a gas environment. Features low humidity levels. The process of rust formation accelerates as the temperature rises.
Radiation
Occurs under intense exposure to radiation. In high-density alloys it occurs slowly.
Underground
If a metal part lies underground for some time, you may notice a green coating or other color distortions on its surfaces. This is a consequence of oxidative processes that occur in different types of soil.
Contact
Appears quickly in places where two different metals come into contact with each other. This is due to the difference in stationary potential in the electrolyte.
Biocorrosion
The process of destruction of metal parts, which is caused by the influence of various microorganisms and their metabolic products.
Rusty shipwrecks
Corrosion by electric shock
Can occur when exposed to stray or external current. The rate at which rust spreads depends on the current strength, duration, and frequency of its impact on metal parts.
Corrosive cavitation
One of the many processes of self-destruction of different types of metals. It starts when exposed to the external environment, mechanical damage.
Stress Corrosion
The process of destruction of alloys, which occurs when mechanical stress interacts with a corrosive environment. This type of corrosion is dangerous for metal structures that are subject to heavy loads.
Fretting corrosion
A complex corrosion process that occurs under the influence of a corrosive environment with various vibrations. To prevent the formation of rust, it is important to reduce the coefficient of friction of metal parts.
By nature of destruction
Kinds:
- solid;
- selective;
- local;
- subsurface;
- intercrystalline;
- slotted.
They differ in location, degree of penetration into the material, and severity of destruction.
Solid
With this corrosion process, all metal surfaces become covered with rust. It can be uniform or uneven, depending on the rate of destruction of the material in different places of the part.
Electoral
A similar process affects one of the elements of the metal structure, which does not have an anti-corrosion coating that slows down the destruction process.
Rusty car (Photo: pixabay.com)
Local
Rust spots are scattered on the metal surface. They are recesses of different sizes, some of which may be superficial, others through.
Subsurface
Appears under metal surfaces. It quickly penetrates deep into the material. This type of corrosion process is characterized by metal delamination.
Intercrystalline
Begins to appear along the boundaries of individual grains of the material. It is extremely difficult to identify by appearance. Indicators of density, strength, and ductility quickly deteriorate. Parts become fragile.
Slotted
Formed at the junction of two metal parts. May appear in technological gaps, under technical gaskets.
Types of corrosion according to the mechanism of the process:
— chemical is a type of corrosion destruction associated with the interaction of a metal and a corrosive environment, in which the metal is simultaneously oxidized and the corrosive environment is restored;
- electrochemical - the process of interaction of a metal with a corrosive environment, in which the reduction of the oxidizing component of the corrosive environment does not occur simultaneously with the ionization of metal atoms and their rates depend on the electrode potential of the metal.
Protection methods
To protect metal surfaces from corrosion, various techniques are used. Each of them is unique and has certain characteristics.
Application of protective coating
Protective coatings can be of two types - metallic and non-metallic. Types of non-metallic coatings:
- Chemical layer. More often these are oxide films that form on the surface under the influence of steam and air. One of the oxidation options is to immerse parts in a solution of nitric acid heated to 140°C.
- Paint and varnish coatings. The main disadvantage of paint and varnish coatings is their low resistance to temperature changes and mechanical damage.
- Powder paints. They are applied using specialized equipment in closed painting booths.
- Various polymer coatings.
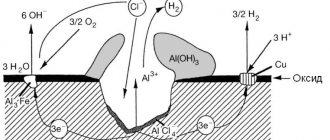
How to prevent electrochemical corrosion?
The protection process begins at the stage of creating a metal object. There are certain operating standards. They are developed based on economic feasibility and safety. A striking example is galvanizing. Galvanized metals are much less susceptible to electrochemical corrosion, but main pipelines are not made from them. This is not economically viable, so other methods, such as insulation, are being developed for pipelines.
A zinc layer on stainless steel heated towel rails is one of the most common methods used to maintain the integrity and protect the surface.
A zinc layer on stainless steel heated towel rails is one of the most common methods used to maintain the integrity and protect the surface.
Alloying is the most common way to increase corrosion resistance. At the stage of creating the alloy, a certain percentage of metals with the least susceptibility to corrosion is added to its composition. Unfortunately, the periodic table of elements does not describe the stability factor, but some patterns can be traced. The least stable are the alkali metals found in groups 1 and 2. However, in the subgroups indicated in blue in the table, there is a connection with atomic number. The higher it is, the more stable the metal:
- copper (29);
- zinc (30);
- silver (47);
- cadmium (48);
- gold (79).
A pattern is also observed in side subgroups 4 and 6:
- titanium (22);
- chromium (24);
- zirconium (40);
- molybdenum (42).
And so on. And the most stable metals are in group 8 (osmium, iridium, platinum), but due to their high cost, they are used extremely rarely in alloying steels.
As for protecting the finished product, there are 4 types, each of which is divided into several methods. For example, metal coatings are divided into:
- diffusion;
- galvanic;
- metallization
The technology for applying the protective layer differs, but they are united by the essence of protection. The metal product is coated with a layer of another metal that is more resistant to electrochemical corrosion. This allows you to maintain the characteristics of the original steel used in the production of the product, but increases the level of protection, since corrosion affects the top layer.
Non-metallic protection methods are also divided into several categories:
- paint and varnish;
- oxide;
- phosphate;
- enamel;
- polymer.
The essence of these methods is to apply a non-metallic component to the surface. They are less expensive, but inferior in quality to metallized types. Any coating has a limited service life, but you can update the coating without significant costs.
The essence of these methods is to apply a non-metallic component to the surface. They are less expensive, but inferior in quality to metallized types. Any coating has a limited service life, but you can update the coating without significant costs.
In addition, there are protection methods that are not related to the product itself. They consist in reducing the aggressiveness of the environment. This may include lowering the humidity level in the room, or adding special inhibitors to the environment, that is, process retarders. Electrical protection is often used with underground structures, directing a negative current charge to the product, thereby turning it into an independent conductor. This protects the product from stray currents, but does not reduce exposure to moisture.
How to protect a heated towel rail from the effects of “stray currents”? Here are some real ways:
- And extremely important. Entrust the installation of a heated towel rail only to professionals with a certain level of qualifications confirming their ability to carry out this type of work!
- Be sure to ground the device. This can be done in several ways. Technically, metal pipes will need to be connected to the PE bus of the electrical panel on the floor using copper wire. For metal-plastic pipes, you will need to install a metal insert, for example, a nipple, between the ball valve and the connection element - connect a copper wire to it and also connect it to the nearest electrical panel. In a combined system, you will need to additionally connect the torn metal parts of the riser with a wire.
- Level potentials within the room. To do this, a special equalization system is used and a box with a plastic case with a grounding bus is installed. All “potentially” current-carrying devices are connected to the bus using a copper cable. The bus itself, which has a larger cross-section, is connected to the floor electrical panel.
- There is a simpler way out of the situation - purchasing a heated towel rail from a seamless pipe, an example of this is a heated towel rail.
- Replace the water heated towel rail with an electric one. All electric heated towel rails have low power, so they can be plugged into a regular electrical outlet. But, since water is constantly present in the bathroom and there is high humidity, the device should be connected only through a residual current device (RCD) and a circuit breaker (circuit breaker). Grounding is also mandatory here!!!
Methods for removing corrosion
If rust has already appeared, it can be removed in different ways - mechanically, chemically. You can also use folk remedies.
Rusty castle (Photo: pixabay.com)
Mechanical cleaning
Involves the use of abrasive tools. Damaged parts will be cleaned by friction.
Brush on metal
It is a classic hand brush with many metal fibers used for cleaning. Suitable for partial removal of the effects of corrosion.
Recommendations
Adviсe:
- It is better not to skimp on protecting parts and cover them with rubber or polymer paint.
- Before using abrasives, you should try to remove rust with gentle compounds.
- Complex corrosion processes can be stopped with the help of aggressive chemicals, but before using them, you need to study the properties of the composition and the characteristics of the metal in order to prevent possible negative reactions.
Immediately after removing rust, surfaces should be coated with a protective compound to reduce the risk of corrosion re-spread.
Corrosive processes can quickly destroy any material. Damage to metal structures in some situations can have catastrophic consequences. Having studied the methods of protection against corrosion, you need to use one of the most suitable ones.
Is it possible to eliminate traces of electrochemical corrosion?
Unfortunately, there is no 100% method of corrosion protection, at least not an economically feasible one. Any product will sooner or later undergo aging, and it will be difficult to get rid of it. If the product begins to rust, you should first determine the cause.
Unfortunately, there is no 100% method of corrosion protection, at least not an economically feasible one. Any product will sooner or later undergo aging, and it will be difficult to get rid of it. If the product begins to rust, you should first determine the cause.
Atmospheric corrosion is most often encountered in everyday life, and the way to eliminate it is by applying non-metallic components, or, more simply, by painting. However, this also has its own nuances, since if traces of corrosion are not eliminated, it will continue to spread under the coating, reducing all efforts to zero.
First you need to eliminate the source of infection. Most of these are superficial lesions that are removed mechanically, that is, by stripping. Difficulties arise with areas of deep penetration, when it is not possible to remove such a layer to eliminate the defect. Also, special attention should be paid to removing the oxide film from the surface. It is the same electrolyte. A simple way is degreasing. Any means with an octane number are used: gasoline, solvent, and so on. This process should not be neglected, since if a film remains on the painted surface, the destruction will continue even under a layer of enamel or polymer.
Better yet, contact a management design engineer. He will tell you the root of the problem and help with its solution.