Various anti-corrosion protection technologies are used for metal sheets and parts. Cathodic protection against corrosion has become widespread. This method has a number of characteristic features, and most often cathodic protection is used for large objects. These could be pipes, cars, metal pile structures, sea vessels. How exactly are pipelines protected from corrosion at the physical and chemical level?
Basic cathodic protection technologies
Cathodic protection is a special method of electrochemical protection of metal objects from rust and corrosion. The main principle is that a negative electrical potential is applied to the metal object being protected. This minimizes metal contact with external ions and substances with an electrical charge. The technology was developed approximately 200 years ago by British scientist Humphry Davy. To confirm his theory, he compiled several reports that were submitted to the government. Based on these reports, the world's first cathodic protection of a large industrial ship was carried out.
Anti-corrosion protection applies to various objects - pipelines, cars, roads, airplanes and so on. Please note that the type of metal does not matter - it can be iron, copper, silver, gold, aluminum, titanium and any other metal, as well as various alloys (with or without alloying additives). Corrosion protection of a car, individual pipe fragments, various decorative items of complex shape, and so on can be equally successful.
1 way
Connecting the part to an external source of electric current (usually compact substations perform this role). When the technology is used, the metal object acts as a cathode, and the electrical substation acts as an anode. Due to this, a shift in the electrical potential occurs, which makes it possible to protect the metal object from electrically active particles. The main areas of application of this technology are the protection of pipelines, welded structures, various platforms, road surface elements, and so on. This technology is quite simple and universal, so it is very popular in the world. Its main disadvantage is the need to connect the protective circuit to an external current source, which can be inconvenient in the case of objects that are located far from human civilization (this problem is partly solved through the use of autonomous energy sources).
Method 2
Galvanic polarization method (galvanic anode technology). This technique is also quite simple and intuitive: a metal object is attached to another that has a negative charge (most often this element is made of light metals - aluminum, zinc, magnesium). Galvanic polarization technology is usually used in cases where there is a protective layer on the surface of the object. This technology is popular in America, where there are a large number of sparsely populated areas and where there is a shortage of external energy sources. Experts argue that galvanic polarization could become very popular in Russia due to the peculiarities of our geography if a protective coating were applied to domestic pipelines (in such a scenario, the use of the first technology would be very difficult, forcing people to look for an alternative).
Areas of application of anodic coatings
Anodic protective coatings are generally suitable for titanium, steels, iron and aluminum. For example, our company has products made of anodized aluminum. We offer both delivery and installation.
This includes:
- pavilions for swimming pools. Anode layers are applied to their frames and rails;
- arenas, hangars and sheds (including trade);
- structures for sports needs (for example, hockey stands);
- advertising profiles;
- railings, handrails and fences;
- elements of windows and doors.
Cathode polarization technology
In this case, the so-called superimposed current is used. An external conductor (often) or a current source (rarely) is used to apply it to a metal object. Upon contact with an electrically active particle, the following happens: the particle, under the influence of electrical attraction forces, moves to a protective element with a negative charge, where “recycling” of these particles occurs.
The consequences of such “disposal” are obvious - the protective element itself becomes corroded over time and becomes unusable. Therefore, this technology is often called the sacrificial electrode method (instead of our part, the “sacrificial electrode” rusts).
In addition to current and voltage, when working with cathodic polarization, one more important parameter must be taken into account - the ohmic voltage. In a technical sense, this parameter reflects the fact that as an electrical charge flows over time, the current voltage in the circuit drops. The drop itself occurs due to the fact that the cathode current flows along a circuit with a lower charge. If the circuit is assembled correctly, this indicator is quite small - thanks to this, the same current of the same power will always be maintained in the circuit.
Products by topic
- Choose …
View
Street bars
27950 ₽ – 41600 ₽
- Choose …
View
Horizontal bar
17550 ₽ – 39000 ₽
- Add to cart
View
Bench with stops
26950 ₽
- Add to cart
View
Swedish wall
35900 ₽
- Add to cart
View
Inclined bench for abdominal exercises
27950 ₽
- Add to cart
View
Workout complex VK-003
151500 ₽
- Add to cart
View
Workout complex VK-001
132500 ₽
- Add to cart
View
Workout complex VK-002
82200 ₽
- Add to cart
View
Workout complex VK-004
137800 ₽
- Add to cart
View
Workout complex VK-005
98800 ₽
- Add to cart
View
Single handle bar
54500 ₽
- Add to cart
View
Parallel bars
9750 ₽
Technology for creating protection stations
Another technology for creating cathodic protection is connecting the element to external current sources. In most cases, for these purposes, special cathodic protection stations (CPS) are built, which consist of several elements - the main current source, anode grounding, various cables and wires connecting individual structural elements and auxiliary points with mechanical or computer control, which allow you to control the parameters .
Most often, this technology is used for objects located near power lines - these can be pipelines, various factory buildings, and so on. VCS can operate in multi-threaded mode - in this case they will serve several protection systems at once. On pipes, a practice has become widespread in which several separate blocks are placed on the pipes to distribute the current more efficiently. The thing is that in the case of long pipelines, in the places where the pipes are connected to current sources, special points with an increased level of electric field voltage are formed - because of this, damage to the pipes can occur. The use of such blocks allows electricity to be distributed evenly throughout the entire protective circuit.
Automation
Checkpoints can operate both manually and automatically:
- In the case of manual control, the change in voltage parameters is regulated by the operator. At the physical level, regulation is carried out by switching the operation of the transformer. The operation of the winding is regulated, which allows you to change the parameters of the electric current.
- In the case of automatic control, the change in voltage parameters is regulated by the device itself based on the parameters that were once set by the operator. At the physical level, control is carried out using special semiconductor thyristors. They turn on or off when the electric current parameters deviate from the specified parameters.
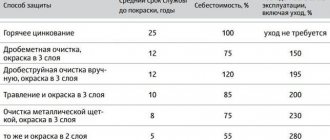
Processing rules
Before applying anticorrosive, it is necessary to prepare the surface. The requirements for surface preparation are as follows.
- Any areas of rust or cracked paint or varnish must be removed.
- The surface must be cleaned of dirt, oils and dried.
- The anti-corrosion coating is applied using a special gun, or with a brush or roller.
At enterprises where the exposure to an aggressive environment is too high, their exposure is reduced by:
- introduction of inhibitors;
- removing connections that are conductors of rust.
There are also SNiPs. Here are some of them.
- Impregnation of metal with a material with high chemical resistance.
- Pasting with special film.
- Use of paints and varnishes, oxide and metallized coatings.
The rules for preventing structures from rusting always indicate the composition of mixtures depending on the area in which the protected products will be used. The compositions can be aggressive, slightly aggressive, or non-aggressive at all.
Local anti-corrosion protection
The rules also specify biologically active or chemically active media. They are also divided into liquid, solid and gaseous.
In any case, painting the product is mandatory, as it gives it not only protective properties against corrosion, but also an external aesthetic appearance.
Features of cathodic protection of pipes
Corrosion in pipelines usually occurs due to various defects and damage to the pipes - ruptures, cracking, cracks, and so on. Due to corrosion, the sealing of pipes is compromised, which can lead to complete or partial failure of the pipeline. This problem is especially acute for underground pipelines. When pipes are placed underground, areas with different electrical potentials are created. This is due to the heterogeneity of the soil and the presence of various debris of inorganic origin in the soil. If there is a serious potential difference, negatively charged ions in the ground begin to react with the metal. This leads to corrosion, which quickly destroys the pipeline.
Electric potential
Cathodic protection of pipelines against corrosion is carried out according to two standard schemes. Using cathodic polarization and by creating external stations. Pipeline protection should be aimed primarily at reducing the rate of destruction of the pipe material. This is done by reducing the electrical potential of the pipe in comparison with the electrical potential of the soil:
- The electrical potential of most modern pipes is approximately 0.8-0.9 volts.
- It has been experimentally shown that the main rocks of the soil have a potential of approximately 0.5-0.6 volts.
To equalize the electrical potentials, it is necessary to reduce the potential of the pipes by only 0.3-0.4 volts. This allows you to almost completely stop the appearance of rust. If the work is carried out correctly, the rate of natural rusting will be less than 1 mm per year.
Choosing a method
The technology for creating external protection stations is suitable for pipes. In this case, overhead power lines with voltages from 500 to 10,000 volts are used as power sources. The higher the voltage, the more pipes can be serviced. Sometimes there are no such lines in a particular area. In this case, it makes sense to install various generators.
External station technology has one major drawback. To create protection, labor-intensive and complex work will have to be carried out. This significantly increases the cost of creating a pipeline. When working with high voltage, excess electrical voltage may be created at the point of electricity supply - this can cause hydrogen cracking of pipes, so when carrying out installation work, electrical wiring must be done carefully.
Instead of the technology of protective stations, you can use the technique of using galvanic anodes to create a polarization effect. This technology is suitable for soils with low resistivity (up to 50 Ohms per 1 sq. m). If the soil resistivity is very high, then the technology of using galvanic anodes is practically useless due to its low efficiency.
Powder coating
An alternative to paint and varnish compositions was powder coating, invented in 1950.
Externally, the application process is similar to spraying with compressed air. However, the absence of “fog”—a suspension of paint in the air—is immediately striking. The part itself seems to attract paint, which settles on it in an even layer.
The attraction provides electricity. The part itself is charged with a positive charge, and the paint with a negative high-voltage charge. And since unlike charges attract, the paint sticks to the metal, just as pieces of paper stick to a comb rubbed against hair.
Then the part is heated to a temperature of 200-250 degrees. The paint melts and, spreading, forms the thinnest, only a few tens of microns, but durable dense layer.
Strict technological requirements and expensive equipment dictate a cost that is one and a half to two times higher than that of conventional paint. However, the high quality of monolithic polymer coatings allows them to compete on equal terms with traditional ones, but even displace them in some cases.
Today, the application of powder coatings has become commonplace; it can even be ordered via the Internet https://oooprofpokraska.ru/metall/.
Features of cathodic protection of cars
Corrosion on cars often appears suddenly. The speed of its spread is very high, since the car has a large number of moving parts. During operation, various small cracks and dents may form in such elements. This significantly increases the risk of corrosion. Cathodic protection of a car against corrosion is usually carried out by redistributing the electrical potential.
Usually special electronic modules are used, which are compact in size and mounted inside the car. Installation of such blocks takes no more than 20 minutes.
Additional processing
It is also worth noting that the cathodic protection method is usually combined with other techniques:
- All main parts of the car are coated with special paints and mastics. They create a protective layer on the metal surface. This layer is electrically neutral. Therefore, upon contact with electrically active substances or ions, rusting does not occur.
- Some vehicle components may be coated with protective cathode plates, which also minimize the risk of rust. Plates are usually used to cover the moving parts that are most likely to crack and become damaged. This is the bottom of the car, rear wheel arches, headlights, interior door surfaces, and so on.
Primer
These compositions are not decorative and should not be used as a finishing coating. The primer only prepares the surface for painting, adding a layer of additional protection.
The use of a primer is quite important, since it performs several functions at once:
- enhances the adhesion of metal to the finishing layer;
- prevents the oxidation process;
- enhances the protective properties of paint;
- reduces the consumption of the main coating material.
There are a great variety of primers for metal on the construction market, but before processing the structure, it is important to understand what problem should be eliminated and what properties of the paint should be enhanced. Let's highlight the main types of primers for metal and describe what they are used for.
The composition of the primer depends on the degree of damage to the metal
Table 1. Types of primers (characteristics).
| Primer type | Brief description of the composition |
| Passivating | They transform the metal into a passive state in relation to the environment. The main active substance is chromates, the nuance is in their quantity in the composition. A low percentage of chromates in the primer will have the opposite effect and speed up the corrosion process. |
| Insulating | Form a thin protective film on the surface of iron. Usually made on an epoxy or alkyd base. A very budget option, recommended for use on ferrous metals. |
| Phosphating | Such primers are essentially passivating, but are suitable for work on non-ferrous metals. In everyday life, they are best known as zinc primers, because they are most often used for processing galvanized iron. |
| Tread | The mixtures contain phosphoric acid and form a protective film. Suitable for working on surfaces damaged by corrosion. |
| Inhibitory | New compounds. They are even produced on a water basis, for this reason they are easy to use. Slow down the process of corrosion that has already begun. |
Using a primer will not only protect the metal from corrosion, but will significantly extend its service life.
Primers for metal are always colored so that the treated area is clearly visible
Types of corrosion
Before you protect metal from rust, you should find out about the existing types. The method of providing anti-corrosion protection is directly dependent on the conditions of use of the parts. Therefore, it is customary to distinguish the following types:
- corrosion, which is associated with atmospheric phenomena;
- destruction of the metal structure in water due to the presence of salts and bacteria in it;
- destructive processes occurring in the soil (soil corrosion).
Methods of anti-corrosion protection must be selected individually, based on the conditions under which the metal product will be used.
As for the types of structural damage, they can be as follows:
- rust is found on the entire surface of the product in separate areas or as a continuous coating;
- has the appearance of spots and penetrates deep into the element;
- destroys metal molecules, leading to cracks;
- large-scale rusting, which destroys not only the surface, but also deeper layers.
Types of destruction can also be combined. In some situations, they are very difficult to identify by eye, especially with spot rusting.
It is customary to emit chemical corrosion. Upon contact with petroleum products, alcohols and other aggressive substances, a special reaction occurs, which is accompanied by high temperature and gas emissions.
During electrochemical corrosion, the surface of a metal alloy comes into contact with water (electrolyte). In this case, diffusion of the material occurs. The electrolyte causes an electric current to occur, and the electrons of the metal are replaced and begin to move, resulting in rust.
Providing corrosion protection and smelting steel products are two interrelated things. Corrosion causes significant damage to commercial or industrial buildings. In addition, this process can lead to disaster, if we talk about, for example, power poles, bridges, barriers, etc.
Typical types of rust damage
The following characteristic types of corrosion damage are distinguished:
- The surface is covered with a continuous rusty layer or individual pieces.
- The part has small areas of rust penetrating into the thickness of the part.
- In the form of deep cracks.
- One of the components in the alloy is oxidized.
- Deep penetration throughout the entire volume.
- Combined.
Types of corrosion damage
Due to their occurrence they are also divided into:
- Chemical. Chemical reactions with active substances.
- Electrochemical. Upon contact with electrolytic solutions, an electric current arises, under the influence of which the electrons of the metals are replaced, and the crystalline structure is destroyed with the formation of rust.