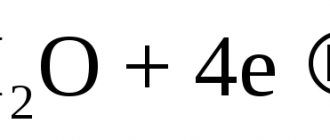Corrosion rate is a multifactorial parameter that depends on both external environmental conditions and the internal properties of the material. In the regulatory and technical documentation there are certain restrictions on the permissible values of metal destruction during the operation of equipment and building structures to ensure their trouble-free operation. In design, there is no universal method for determining corrosion rate. This is due to the complexity of taking into account all factors. The most reliable method is to study the operating history of the facility.
Criteria
You might be interested in:Isobaric, isochoric, isothermal and adiabatic processes for an ideal gas
Currently, several corrosion rate indicators are used in equipment design:
- According to the direct method of assessment: reduction in the mass of a metal part per unit surface - weight indicator (measured in grams per 1 m2 per 1 hour); depth of damage (or permeability of the corrosion process), mm/year; the amount of released gas phase of corrosion products; the length of time during which the first corrosion damage appears; the number of corrosion centers per unit surface area that appeared over a certain period of time.
- According to indirect assessment: current strength of electrochemical corrosion; electrical resistance; change in physical and mechanical characteristics.
You may be interested in: Such ordinary people, or the meaning of “why not”
The first indicator using the direct assessment method is the most common.
Depth corrosion index
∏=8.76•v/ρ , where
8.76 is the coefficient for the transition from measuring the weight indicator of the corrosion rate per 1 hour to the depth indicator per 1 year (24 hours • 360 = 8760 hours);
v—corrosion rate, g/(m2•h);
ρ—density, g/cm3;
If corrosion is local in nature, its speed cannot be accurately characterized by weight or depth. For pitting corrosion, it is necessary to determine the maximum depth index. With intergranular corrosion and corrosion cracking, the corrosion rate is quantitatively characterized by a mechanical corrosion indicator , for example, by loss of strength:
Kσ=(σ0–σ1/σ0)•100% , where
σ0 —ultimate strength before corrosion;
σ1 is the ultimate strength after corrosion, calculated in relation to the initial cross-sectional area of the metal sample;
Calculation formulas
In the general case, weight losses that determine the rate of metal corrosion are found using the following formula:
Vkp=q/(St),
where q is the reduction in metal mass, g;
S – surface area from which the material was transferred, m2;
t – time period, hours.
For sheet metal and shells made from it, the depth indicator is determined (mm/year):
H=m/t,
m is the depth of penetration of corrosion into the metal.
There is the following relationship between the first and second indicators described above:
H=8.76Vkp/ρ,
where ρ is the density of the material.
Scale for rough assessment of corrosion resistance of metals
| Durability group | Depth corrosion rate, mm/year | Point |
| Absolutely resistant | 0,001 | 1 |
| Very resistant | 0,001 … 0,005 | 2 |
| 0,005 … 0,010 | 3 | |
| Persistent | 0,01 … 0,05 | 4 |
| 0,05 … 0,10 | 5 | |
| Low resistant | 0,1 … 0,5 | 6 |
| 0,5 … 1,0 | 7 | |
| Low-resistant | 1,0 … 5,0 | 8 |
| 5,0 … 10,0 | 9 | |
| Unstable | 10,0 | 10 |
Main factors influencing the corrosion rate
You might be interested in: Comic nominations for teachers for graduation
The rate of metal destruction is influenced by the following groups of factors:
- internal, related to the physical and chemical nature of the material (phase structure, chemical composition, surface roughness of the part, residual and operating stresses in the material, and others);
- external (environmental conditions, speed of movement of a corrosive environment, temperature, atmospheric composition, presence of inhibitors or stimulants, and others);
- mechanical (development of corrosion cracks, destruction of metal under the influence of cyclic loads, cavitation and fretting corrosion);
- design features (choice of metal grade, presence of gaps between parts, roughness requirements).
Physicochemical characteristics
The most important among internal corrosion factors are the following:
- Thermodynamic stability. To determine it in aqueous solutions, Pourbaix reference diagrams are used, the abscissa of which is the pH of the medium, and the ordinate is the redox potential. A positive shift in potential means greater stability of the material. It is roughly defined as the normal equilibrium potential of the metal. In reality, materials corrode at different rates.
- Atom position in the periodic table of chemical elements. The metals most susceptible to corrosion are alkali and alkaline earth metals. The rate of corrosion decreases as the atomic number increases.
- Crystal structure. It has an ambiguous effect on destruction. The coarse-grained structure itself does not lead to an increase in corrosion, but is favorable for the development of intercrystalline selective destruction of grain boundaries. Metals and alloys with a uniform phase distribution corrode uniformly, while those with a non-uniform phase distribution corrode according to a focal mechanism. The mutual arrangement of the phases performs the function of anode and cathode in an aggressive environment.
- Energy heterogeneity of atoms in a crystal lattice. Atoms with the highest energy are located in the corners of the edges of microroughness and are active centers of dissolution during chemical corrosion. Therefore, careful mechanical processing of metal parts (grinding, polishing, finishing) increases corrosion resistance. This effect is also explained by the formation of denser and more continuous oxide films on smooth surfaces.
Influence of acidity of the environment
You might be interested in: Dig or drip? How to write correctly?
During the process of chemical corrosion, the concentration of hydrogen ions affects the following points:
- solubility of corrosion products;
- formation of protective oxide films;
- metal destruction rate.
At a pH in the range of 4-10 units (acidic solution), corrosion of iron depends on the intensity of oxygen penetration to the surface of the object. In alkaline solutions, the corrosion rate first decreases due to surface passivation, and then, at pH>13, increases as a result of the dissolution of the protective oxide film.
Each type of metal has its own dependence of the intensity of destruction on the acidity of the solution. Noble metals (Pt, Ag, Au) are resistant to corrosion in acidic environments. Zn and Al are quickly destroyed in both acids and alkalis. Ni and Cd are resistant to alkalis, but easily corrode in acids.
Classification of corrosion processes.
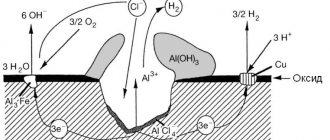
- According to the mechanism of the process, a distinction is made between chemical and electrochemical corrosion of metal. Chemical corrosion is the interaction of metals with a corrosive environment, in which the metal is oxidized and the oxidizing components of the corrosive environment are reduced in one act. This is how the oxidation of most metals occurs in gaseous environments containing an oxidizing agent (for example, oxidation in air with increasing temperature). Electrochemical corrosion is the interaction of a metal with a corrosive environment, in which the ionization of metal atoms and the reduction of the oxidizing component of the environment does not occur in an aqueous act, and their rates depend on the electrode potential of the metal. This process involves, for example, the interaction of a metal with acids.
- According to the nature of corrosion destruction. General or continuous corrosion in which the entire surface of the metal is corroded. It is accordingly divided into uniform (1a), non-uniform (1b) and selective (1c), in which the corrosion process spreads predominantly over any structural component of the alloy. Local corrosion in which certain areas of the metal corrode: pitting corrosion - corrosion damage in the form individual medium and large stains (corrosion of brass in sea water)
- intercrystalline corrosion, in which the corrosion process spreads along the metal-alloy boundary (aluminum is alloyed with chromium-nickel) and other types of corrosion.
- Gas corrosion is corrosion in a gas environment at high temperatures. (liquid metal, during hot rolling, stamping, etc.)
1.2 Corrosion rate indicator.
To establish the rate of corrosion of a metal in a given environment, observations are usually made of changes over time in some characteristic that objectively reflects a change in the properties of the metal.
The following indicators are most often used in corrosion practice.
- Mass change indicator - change in the mass of a sample as a result of corrosion per unit of metal surface S and per unit of time (for example, g/m h) depending on the corrosion conditions, they are distinguished: negative indicator of mass change
- positive mass change indicator
the oxygen (K) and hydrogen (K) index are introduced respectively. The hydrogen index of corrosion is the volume of H released during the corrosion process, referred to Su. The oxygen index of corrosion is the volume of O absorbed in the process, divided by Su.
where Ro and R are the electrical resistance of the sample before and after corrosion, respectively.
This method has some drawback: the thickness of the metal must be the same throughout the test, and for this reason the resistivity is most often determined, i.e. change in electrical resistance per unit area of the sample (cm, mm) with a length equal to one. This method has limitations of application (for sheet metal no more than 3 mm). The most accurate data are obtained for wire samples. This method is not suitable for welded joints.
Composition and concentration of neutral solutions
The rate of corrosion in neutral solutions depends to a large extent on the properties of the salt and its concentration:
- The hydrolysis of salts in a corrosive environment produces ions that act as activators or retarders (inhibitors) of metal destruction.
- Those compounds that increase pH also increase the rate of the destructive process (for example, soda ash), and those that reduce acidity reduce it (ammonium chloride).
- In the presence of chlorides and sulfates in the solution, destruction is activated until a certain salt concentration is reached (which is explained by the intensification of the anodic process under the influence of chlorine and sulfur ions), and then gradually decreases due to a decrease in oxygen solubility.
Some types of salts are capable of forming a poorly soluble film (for example, iron phosphate). This helps protect the metal from further destruction. This property is used when using rust neutralizers.
Basic indicators of corrosion and methods for assessing corrosion resistance
4>
Corrosion causes mass changes; mechanical, electrical, optical, electrochemical properties; composition and structure, state of the metal surface. These changes are characterized by corrosion resistance
(chemical resistance of materials).
Corrosion resistance is the ability of a metal to resist the corrosive effects of the environment.
Corrosion resistance is determined qualitatively and quantitatively - based on corrosion indicators (corrosion rate in given conditions, time to achieve a given degree of corrosion damage), semi-quantitatively - by a group or resistance score on a 10-point scale adopted in the CIS. Based on these indicators, the service life, durability and reliability of metal structures are determined.
Corrosion effects or integral indicators of corrosion correspond to speed or differential indicators
.
Differential indicators are found as the ratio of changes in the corrosion effect or the first derivative of time by graphical or analytical methods.
Towards qualitative indicators of corrosion
relate:
— visual assessment of corrosion resistance
— observation of the appearance of samples with photographs, sketches or brief descriptions and observation of changes in the corrosive environment;
— micro-studies in order to establish the nature of corrosion destruction, the presence or absence of local corrosion lesions (intergranular, pitting corrosion, stress-corrosion cracking, etc.);
- the use of special reagents that cause coloring of the anodic and cathodic areas of the corroding metal surface.
Among quantitative indicators, the direct and most reliable way to determine corrosion resistance is the gravimetric (weight) method,
based on changes in the mass of samples during testing.
The gravimetric method determines the corrosion rate — corrosion loss per unit of metal surface per unit of time.
(The rate of corrosion, or the rate of metal ionization reaction, is determined by the number of gram particles reacting on the metal surface per unit time. Since the metal surface has a certain microrelief, the “visible” surface area S, calculated from geometric dimensions, is less than the true surface S0, on which interaction of the metal with the corrosive environment occurs. These areas are related by the relation: , where q is the roughness coefficient. For a perfectly smooth surface (for example, liquid metal), the coefficient is equal to unity and S = S0. For hard metals q> 1. The values of S0 and q are difficult to establish experimentally, therefore, the “visible” surface area S is usually used in calculations, and the number of reacted atoms is replaced by the mass of the oxidized metal.)
Depending on the state of the corrosion products, various versions of the gravimetric method are used. The specific mass loss per unit area is determined by the formula:
, (1.2) where m0
– mass of the sample before testing [
kg,
g],
mτ –
mass of the sample after testing and removal of corrosion products [m2]. Typically, specific mass loss is expressed in . To calculate the mass loss from the increase in mass of the sample, it is necessary to know the composition of the corrosion products.
When easily removable corrosion products are formed (by mechanical, chemical or electrochemical methods), a negative mass change indicator is used,
or
corrosion rate under given conditions :
, (1.3) where m0 and mτ are the mass of the sample before and after corrosion tests and removal of corrosion products, respectively, [g]; S –
area of the oxidized metal surface, [m2];
τ –
test time, [h]. (This method makes it possible to determine the rate of continuous, uniform and uneven, local and subsurface corrosion).
If hard-to-remove corrosion products are formed on the metal surface, well adhered to the surface, then a positive indicator
corrosion rates:
, (1.4) where m0
and
mτ –
mass of the sample before and after corrosion tests together with corrosion products, respectively, g;
S –
area of the oxidized metal surface, m2;
τ –
test time, h.
Having determined the composition of metal corrosion products, you can recalculate a positive indicator of mass change into a negative one and vice versa:
, (1.5) where AMe
,
Aok
– mass of gram-atom of metal and oxidizing agent, respectively;
nMe
and
nok
are the valency of the metal and the oxidizing agent, respectively.
Another direct and widely used method for determining corrosion resistance in practice is the corrosion penetration rate or depth index.
corrosion
KH
:
, (1.6)
where H is the depth of corrosion destruction (corrosion penetration) [mm], determined by direct measurement (calipers, micrometer, microscope, ultrasonic inspection, etc.), per unit time - τ
[h].
(With continuous corrosion at a constant rate, the indicator is called linear corrosion rate , .
Deep indicators are more universal in nature and they determine the rate of local corrosion).
Knowing the negative and depth indicators, it is possible to determine the time before the mass per unit area decreases by the permissible amount ( ), and the time for corrosion to penetrate to the permissible depth ( ).
With uniform corrosion, the negative indicator (in g/m2.h) and the depth indicator are related by the ratio:
, (1.7) where dMe
– metal density, [g/cm3].
To qualitatively and quantitatively assess the corrosion resistance of a metal under specific conditions, it is recommended to use the scale of corrosion resistance of metals (Appendix, Table 1), in which the unit of corrosion resistance is a point. The scale has 10 points and is comparative in nature.
Volumetric (volumetric) method
is based on measuring the volume of gas released as a result of corrosion and allows one to determine
the hydrogen index of corrosion (the volume of hydrogen released as a result of the corrosion reaction) and the oxygen index (the volume of oxygen absorbed as a result of corrosion).
Volume indicators represent the ratio of the volume ΔV of released or absorbed gas from a unit surface per unit of time, reduced to normal conditions (T=273 K, P=1.013·105 Pa): , (1.8)
The volumetric corrosion index is associated with a positive ratio:
, (1.9) where MGi VM is the molecular weight and volume of a mole of gas (22400 cm3) under normal conditions; 104 – conversion factor cm3
in
m3
.
Integral corrosion indicators include mechanical corrosion indicators, KM
, the ratio of the change in mechanical properties before and after corrosion in% (for example, strength):
,
(1.10)
where Δ σВ
=
σ
–
σВ
,
σ
and
σВ
are the tensile strength of the metal before and after corrosion tests, respectively.
Mechanical indicators are used to evaluate intergranular corrosion, corrosion cracking, corrosion embrittlement ,
and so on.
Change in electrical resistance
metal when corroded over a certain time is used as an indicator of corrosion:
, (1.11)
where ΔR=Rτ – R0
,
R0
and
Rτ
– electrical resistance of the sample before and after corrosion tests during time
τ
.
This indicator can be used both for general corrosion and local (intergranular, pitting). When using this indicator, it is important to take into account that the electrical resistance depends on the structural state of the metal (alloy), so it should not change during corrosion tests (for example, the decomposition of a solid solution of any alloy component during high-temperature tests).
Other indicators may be reflectivity deterioration, time until the first corrosion lesion appears, etc.
For local types of corrosion (pitting, spot corrosion, exfoliating corrosion), as well as for corrosion of coated products, the degree of surface damage over a certain time or per unit of time, or a focal corrosion indicator is used as indicators of corrosion resistance
:
KS
= , (1.12)
where Si
and
ni
– area and number of corrosion lesions.
To assess the corrosion resistance of coatings and pitting corrosion, the area of corrosion lesions is determined for each standard size of the corrosion focus, followed by ranking of the results and evaluation on a special 10-point scale of corrosion resistance, in which points are assigned depending on the degree and number of corrosion lesions.
The rate of electrochemical corrosion can be expressed as the current corrosion index i
– anodic current density corresponding to the speed of a given corrosion process – used to study electrochemical corrosion.
The connection between the value i
(A/cm2) and the negative indicator of mass change (g/m2 h) is established on the basis of Faraday’s law:
, (1.13)
where n
– valency of the metal passing into solution;
F = 26.8 Ah/g-eq – Faraday’s constant, AMe
– atomic mass of the metal, g; 104 – conversion factor.
This indicator is determined on the basis of polarization curves or directly - with corrosion meters (for example, such as “KM-MISiS”, etc.) with automatic conversion to depth or weight indicators.
4>
Date added: 2017-02-13; views: 11168; ORDER A WORK WRITING
Find out more:
Corrosion inhibitors
Corrosion retarders (or inhibitors) differ in their mechanism of action on the redox process:
- Anode. Thanks to them, a passive film is formed. This group includes compounds based on chromates and dichromates, nitrates and nitrites. The last type of inhibitors is used for inter-operational protection of parts. When using anodic corrosion inhibitors, it is necessary to first determine their minimum protective concentration, since addition in small quantities can lead to an increase in the rate of destruction.
- Cathode. The mechanism of their action is based on reducing the oxygen concentration and, accordingly, slowing down the cathodic process.
- Shielding. These inhibitors isolate the metal surface by forming insoluble compounds that are deposited as a protective layer.
The last group includes rust neutralizers, which are also used to remove oxides. They usually contain orthophosphoric acid. Under its influence, phosphating of the metal occurs - the formation of a durable protective layer of insoluble phosphates. Neutralizers are applied with a spray or roller. After 25-30 minutes the surface becomes white-gray. After the composition has dried, paint and varnish materials are applied.
Mechanical impact
Increased corrosion in an aggressive environment is facilitated by such types of mechanical impact as:
- Internal (during molding or heat treatment) and external (under the influence of externally applied load) stresses. As a result, electrochemical heterogeneity occurs, the thermodynamic stability of the material decreases, and corrosion cracking occurs. Destruction occurs especially quickly under tensile loads (cracks form in perpendicular planes) in the presence of oxidizing anions, for example, NaCl. A typical example of devices susceptible to this type of destruction are parts of steam boilers.
- Alternating dynamic effects, vibrations (corrosion fatigue). There is an intensive decrease in the fatigue limit, multiple microcracks are formed, which then merge into one large one. The number of cycles before failure largely depends on the chemical and phase composition of metals and alloys. Pump axles, springs, turbine blades and other equipment elements are susceptible to such corrosion.
- Friction of parts. Rapid corrosion is caused by mechanical wear of protective films on the surface of the part and chemical interaction with the environment. In liquid, the rate of destruction is lower than in air.
- Cavitation impact. Cavitation occurs when the continuity of fluid flow is disrupted as a result of the formation of vacuum bubbles, which collapse and create a pulsating effect. As a result, deep local damage occurs. This type of corrosion is often observed in chemical apparatus.
Design factors
When designing elements operating in aggressive conditions, it must be taken into account that the corrosion rate increases in the following cases:
- upon contact of dissimilar metals (the greater the difference in electrode potential between them, the higher the current strength of the electrochemical destruction process);
- in the presence of mechanical stress concentrators (grooves, grooves, holes, etc.);
- with low cleanliness of the treated surface, since in this case local short-circuited galvanic pairs arise;
- when there is a significant difference in the temperature of individual parts of the apparatus (thermogalvanic elements are formed);
- in the presence of stagnant zones (cracks, gaps);
- during the formation of residual stresses, especially in welded joints (to eliminate them, it is necessary to provide heat treatment - annealing).
Assessment methods
There are several ways to assess the rate of destruction of metals in aggressive environments:
- Laboratory - testing samples in artificially simulated conditions close to real ones. Their advantage is that they can reduce research time.
- Field – carried out in natural conditions. They take a long time. The advantage of this method is to obtain information about the properties of the metal under further operating conditions.
- Full-scale – testing of finished metal objects in a natural environment.
Source
Laboratory measurement methods and corrosion control instruments
Corrosion indicators
To quantify the corrosion rate, corrosion indicators are used: massometric, volumetric mechanical, current, etc. The massometric indicator of corrosion rate is the change in the mass of the metal as a result of corrosion per unit of its surface, per unit of time is calculated by the formula.
Where is the mass of the original sample, g; — mass of the sample after testing and removal of corrosion products, g; — surface area of the sample, m2; — test time, h.
Methods for determining the corrosion rate by mass loss are used to assess uniformity. These methods cannot evaluate uneven corrosion, intergranular and transgranular corrosion damage.
The volumetric corrosion index characterizes the volume V of gas released or absorbed in the process of corrosion, reduced to normal conditions (T = 273 K, Pa) and related to a unit of time
Where is the volume of absorbed or released gas, cm2; sample surface area; test time, hours
When metal passes into corrosion products and acidic environments, an equivalent amount of hydrogen is released. In neutral oxygen-containing environments, during the formation of corrosion products, an equivalent amount of oxygen is absorbed. The volume of hydrogen released or oxygen absorbed is measured using a eudiometer.
The depth indicator of corrosion rate takes into account the decrease in metal thickness due to corrosion, expressed in linear units and per unit of time. The average value of the depth of corrosion damage for uniform corrosion can be calculated using the massometric indicator of corrosion rate;
.
Where is the density of the metal, g/cm3; 8.76 is the conversion factor.
This indicator is convenient for comparing the corrosion rate of metals with different densities.
The mechanical corrosion indicator characterizes the change in any indicator of the mechanical properties of the metal (%) over a certain test time.
For example, the indicator of change in tensile strength is determined by the formula
Where is the strength limit of the metal before corrosion, MPa/m2; fictitious ultimate strength after corrosion during the test. MPa/m2.
The current corrosion indicator allows the amount of corroded metal according to the Faraden formula, if the strength of the corrosion current is known
Where is the corrosion current and Faraday's constant; valency of the metal in a given corrosion process; A is the atomic mass of the metal, g; time, s; sample surface area, m2.
When qualitatively and quantitatively assessing the corrosion resistance of metals, it is recommended to use a ten-point scale (GOST 13819–68) (Table 1)
Table 1
Ten-point scale of corrosion resistance of metals (GOST 13819–68)
| Durability group | Metal corrosion rate, mm/year | Point |
| Absolutely resistant | Less than 0.001 | 1 |
| Very resistant | 0.001 to 0.005 | 2 |
| 0.005 to 0.01 | 3 | |
| Persistent | From 0.01 to 0.05 | 4 |
| From 0.05 to 0.1 | 5 | |
| Reduced resistance | From 0.1 to 0.5 | 6 |
| From 0.5 to 1.0 | 7 | |
| Low-resistant | From 1.0 to 5.0 | 8 |
| From 5.0 to 10.0 | 9 | |
| Unstable | From 10 and above | 10 |
Chemical composition and mechanical properties of some structural materials. Steels and cast irons are most widely used for the manufacture of equipment for the oil and gas industry. Among the steels, the most common ones are carbon, low-alloy and stainless steel.
Structural carbon steels are divided into ordinary quality carbon steel and high-quality steel.
Carbon steel of ordinary quality is divided depending on the purpose and guaranteed characteristics into three groups: group A - steel with guaranteed mechanical properties (used for unwelded loaded structural elements); group B - steel with a guaranteed chemical composition (used for welded elements of non-critical structures); group B-steel with guaranteed mechanical properties and chemical composition (used for welded elements of critical structures).
table 2
Chemical composition of ordinary quality carbon steel
| Steel grades | Contents of elements% | ||
| Carbon | Manganese | Silicon | |
| BSt1kp | 0,06–0,12 | 0,25–0,50 | No more than 0.05 |
| BSt1ps | 0,06–0,12 | 0,25–0,50 | 0,05–0,17 |
| BSt1sp | 0,06-,012 | 0,25–0,50 | 0,12–0,30 |
| BSt2kp | 0,09–0,15 | 0,25–0,50 | No more than 0.07 |
| BSt2ps | 0,09–0,15 | 0,25–0,50 | 0,05–0,17 |
| BSt2sp | 0,09–0,15 | 0,25–0,50 | 0,12–0,30 |
| BSt3kp | 0,14–0,22 | 0,30–0,60 | No more than 0.07 |
| BSt3ps | 0,14–0,22 | 0,40–0,65 | 0,05–0,17 |
| BSt3sp | 0,14–0,22 | 0,40–0,65 | 0,12–0,30 |
| BSt3Gps | 0,14–0,22 | 0,80–1,10 | No more than 0.15 |
| BSt4kp | 0,18–0,27 | 0,40–0,70 | No more than 0.07 |
| BSt4kp | 0,18–0,27 | 0,40–0,70 | 0,05–0,17 |
| BSt4kp | 0,18–0,27 | 0,40–0,70 | 0,12–0,30 |
| BSt5ps | 0,28–0,97 | 0,50–0,80 | 0,05–0,17 |
| BSt5Gps | 0,22–0,30 | 0,80–1,20 | No more than 0.15 |
| BSt6ps | 0,38–0,49 | 0,50–0,80 | 0,05–0,17 |
| BSt6sp | 0,38–0,49 | 0,50–0,80 | 0,15–0,35 |
Laboratory measurement methods
Laboratory tests are typically accelerated tests conducted under specified, controlled conditions, which may differ from those found in practice.
Laboratory corrosion tests are used: when studying the mechanism; to evaluate the durability of structural materials and the effectiveness of various methods of corrosion protection.
Preparation of samples. For gravimetric corrosion tests, the sample dimensions are limited so that they can be weighed on an analytical balance. Typically, sample sizes are 40x20x2 or 50x20x2 mm. To fasten the samples during testing, holes with a diameter of 5 mm are drilled in one of the edges of the plate.
Prepared samples are tested according to the method provided by the program: wetting the surface of the sample after immersing it; prepare at least three samples. Increasing the number of samples tested in parallel makes it possible to increase the reliability of the results, especially when it is necessary to establish a relatively small difference in the characteristics of the material.
To obtain reliable information about the change in corrosion rate over time, it is necessary to install such a number of sets of samples for testing to provide at least three test periods.
Removal of corrosion products . Corrosion products are removed after keeping the samples in solutions that interact predominantly with corrosion products
Visual observation of corrosion lesions makes it possible to record changes in the appearance of the metal surface, while noting the time when corrosion products began to appear. Their distribution over the metal surface can be recorded by sequential photography.
To observe the distribution of cathode and anodic areas over the metal surface, special reagents are introduced into the electrolytes. Using K3Fe(CN)6 * 2H2O, it is possible to identify the anodic areas of the surface by the blue coloration of the solution adjacent to them. Phenolphthalein, introduced into a solution, under the influence of an alkaline reaction on the cathode metal surface is painted in a single color.
The depth of a pinpoint lesion is determined using an optical microscope by focusing it first on the undamaged surface and then on the bottom of the pitting.
The gravimetric method is one of the most common methods for determining corrosion rates. The simplest and most accessible method of testing in electrolytes is the open vessel test. In laboratory studies, a minimum of 150 ml of solution is usually used per 1 cm2 of sample surface.
Samples prepared for testing are suspended on glass hooks or nylon threads, lowered into vessels with a medium and tested with complete partial or variable immersion in a stationary or stirred electrolyte, through which air, oxygen, nitrogen or other gas can be passed.
Rice. 1. Scheme of corrosion tests of samples in an open vessel with complete (a), partial (b), immersion and stationary and stirred (c) solution; 1-test sample; 2-suspension; 3- glass beaker with a corrosive solution; 4- stirrer.
Rice. 2. Scheme of operation of a potentiostat in the mode of maintaining a constant polarizing current: 1-source of setting voltage; 2-amplifier; 3- milliammeter; 4- katukna; 5 - working electrolyte; 6-auxiliary electrode; 7- voltmeter; 8- electrochemical cell; 9- reference electrode.
Literature:
- Saakiyan L. S., Efremon A. P. et al. Protection of oilfield equipment from corrosion. M.: - Nedra, 1985, 206 p.
- Kats N. G., Starikov V. P., Parfenov S. N. Chemical resistance of materials and protection of oil and gas processing equipment from corrosion. M.: Mechanical engineering. -2011
- Zhuk N.P. Course on the theory of corrosion and protection of metals: textbook. Manual / Zhuk N.P. -2nd ed., stereotypical. Reprint of the 1976 edition.-M:. LLC TID "Alliance", 2006.-472.