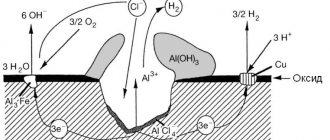
Contact corrosion occurs when two dissimilar metals come into direct contact. It is impossible, for example, to connect aluminum sheets with a copper rivet, since under certain conditions they form a strong galvanic couple.
Different metals have different electrode potentials. In the presence of an electrolyte, one of them acts as a cathode and the other as an anode. As a result of the chemical reaction occurring between them, a corrosion process will begin in which copper (cathode) will mercilessly destroy aluminum (anode).
Almost all pairs of dissimilar metals that are in contact with each other are subject to corrosion, since even moisture from the air can act as an electrolyte and activate their electrode potential. But some couples are more vulnerable, while others are less vulnerable.
For example, aluminum has excellent contact with galvanized steel, chromium and zinc, but brass is not at all “friendly” with steel, aluminum and zinc. To find out which metals are compatible and which are not, let's look at the basics of chemistry.
In the series of electrochemical activity, metals are in the following sequence:
Electrochemical voltage series of metals
For example, consider a pair of aluminum and copper. Aluminum is in the row to the left of hydrogen and has an electronegative potential of -1.7V, and copper is to the right and has a positive potential of +0.4V. A large potential difference leads to the destruction of more active aluminum. Copper is stronger than all the elements in front, so if paired with any of them, it will emerge victorious. The farther apart the elements are in a row, the higher their incompatibility and the likelihood of galvanic corrosion.
Data on the compatibility of some metals are presented in the table:
| Aluminum | Brass | Bronze | Copper | Cink Steel | Iron | Lead | Stainless steel | Zinc | |
| Aluminum | D | N | N | N | D | ABOUT | ABOUT | D | D |
| Copper | N | ABOUT | ABOUT | D | ABOUT | N | ABOUT | N | N |
| Cink Steel | D | ABOUT | ABOUT | ABOUT | D | ABOUT | D | ABOUT | D |
| Lead | ABOUT | ABOUT | ABOUT | ABOUT | D | D | D | ABOUT | D |
| Stainless steel | D | N | N | N | ABOUT | ABOUT | ABOUT | D | N |
| Zinc | D | N | N | N | D | N | D | N | D |
D
– absolutely acceptable contacts (low risk of GC);
O
– limited permissible contacts (average risk of GC);
N
– unacceptable contacts (high risk of GC).
The table below can serve as a quick reference for determining the compatibility of certain structural metals. The permissibility and impermissibility of contacts between electrochemically dissimilar metals is established by GOST 9.005-72.
Example of unacceptable galvanic pairs:
Galvanic action can occur if a stainless steel building structure is fastened with galvanized bolts. In this unwanted pair, the high anode fastener will suffer as its electrons move towards the cathode stainless steel. Therefore, fasteners must be made of a less galvanically active metal than the metal structure material.
The rate of galvanic corrosion is influenced by the surface area of the anode and cathode. If a large anode is connected to a small cathode, the anode will rust slowly, but if the opposite is done, it will rust quickly. For example, use stainless steel bolts to fasten aluminum, but not vice versa.
The intensity of contact corrosion also depends on the operating conditions of the joint. Under normal atmospheric conditions, the process will proceed less quickly and increases in an aggressive electrically conductive environment, for example, solutions of acids and alkalis. The presence of other substances in water increases the conductivity of the electrolyte and the rate of corrosion. Therefore, environmental assessment is important when designing structures.
Types of stainless steel corrosion
Although high-alloy steels are called stainless steels, they are susceptible to corrosion under certain conditions. Let's consider the types of corrosion of stainless steel products, as well as methods of its protection.
Crevice corrosion of stainless steels.
Crevice corrosion is the second most common type of damage to stainless steels after pitting corrosion.
Crevice corrosion occurs in places where a small gap forms between a steel product and another object. This second item is usually an insulating material: a seal or rubber gasket, although it can also be a metal object. The geometry of the gap is a decisive factor in the onset of crevice corrosion. The gap must be large enough to allow penetration of chemically aggressive liquids, but not so large that material can be washed out of the gap by flow or convection of the liquid.
The mechanism of crevice corrosion formation is well known. The first stage is the accumulation of aggressive ions (such as chloride ions) in the gap and the displacement of oxygen from the solution inside the gap. This causes the anode to form in the gap, and the material outside the gap becomes the cathode. Corrosion forms in the gap for two reasons: firstly, the passive film is destroyed due to the displacement of oxygen, and secondly, corrosion reactions in the anodic zone cause a change in the acidity of the environment (over time, the acidity in the gap increases).
Proper design is one of the best ways to avoid crevice corrosion. The choice of materials is comparable in importance. Crevice corrosion is most intense in acidic conditions, in chloride-containing non-fluid media. Cathodic protection can reduce both pitting and crevice corrosion by increasing the alkalinity of the anodic site. Increasing the fluidity of the medium will also reduce the effects of both forms of local corrosion.
Other passive materials, such as aluminum and its alloys, are susceptible to both pitting and crevice corrosion. Pitting and crevice corrosion of aluminum occurs in a similar way to corrosion of stainless steel.
Pitting corrosion
Pitting is a type of extremely narrowly localized corrosion that leads to the formation of small holes in the metal. The driving force behind pitting is a lack of oxygen in a small area. This zone becomes anodic, while the zone of excess oxygen becomes cathodic, causing highly localized galvanic corrosion. This type of corrosion tends to penetrate deep into the metal. Limited ion diffusion maintains local oxygen deficiency. This type of corrosion is very insidious because it does not cause significant damage to the surface of the metal, while deeply damaging its structure. Pitting on the metal surface is often hidden by corrosion products.
The development of pitting begins with a small surface defect: a scratch, a local change in composition, or damage to the protective coating. Polished surfaces exhibit greater resistance to pitting corrosion if polishing has been done correctly. Poor polishing can accelerate the development of corrosion.
Typically, those alloys whose corrosion resistance is ensured by the surface layer are most susceptible to pitting corrosion: stainless steels, nickel alloys, aluminum alloys. Metals that are subject to uniform corrosion usually do not suffer from pitting. For example, ordinary carbon steel in seawater will corrode uniformly, while stainless steel will experience pitting. The addition of about 2% molybdenum increases the resistance of stainless steels to pitting corrosion. The presence of chlorides (eg in seawater) significantly increases the formation and growth of pitting through an autocatalytic process. Standing water also promotes pitting.
Pitting is the most common type of corrosion attack on stainless steel, causing holes in tanks, tanks and pipe walls. It occurs in the form of small in diameter but deep cavities (pittings). Their diameter usually does not exceed 1 mm, but penetration into the depth of the metal can be great.
In a corrosion reaction, pittings act as anodes, and the remaining surface serves as a cathode. The formation of pitting starts with damage to the protective oxide film (passive layer) on the steel surface. Typically, these damages are the inclusion of foreign impurities, such as sulfur, in the steel. Foreign inclusions can lead to a local shortage of alloying elements, thereby disrupting the uniformity of the protective oxide layer.
Favorable conditions for pitting corrosion are moderately high temperatures, high concentrations of chloride ions and other halides (fluorides, bromides, iodides). Acidic environments also promote the development of pitting, which is itself acidic.
The acidity inside the pitting is the reason why, once formed, they continue to grow deeper.
Pitting Resistance Equivalent Numerical (PREN)
The Resistance to Pitting Equivalent Numeric (RREN) is a useful reference that reflects the pitting tendency of certain stainless steels. It should be used as a guide only and not as a guaranteed way to predict corrosion resistance in all circumstances. Alloys having high concentrations of nitrogen (N), chromium (Cr) and molybdenum (Mo) have been found to exhibit high resistance to pitting corrosion. The comparative effectiveness of the combination of these elements is expressed by the following formula:
PREN = (%Cr) + (3.3 x %Mo) + (16 x %N) (note that some options use 32 x %N)
The higher the PREN value, the higher the resistance to pitting corrosion.
Typical PREN values are:
| steel grade | PREN |
| 430 | 16 |
| 444 | 25 |
| 304 | 19 |
| 304LN | 21 |
| 316 | 26 |
| 316LN | 27.5 |
| 904L | 36 |
| Zeron 100 | 41 |
| SAF 2507 | 42 |
Passivation of stainless steel.
For applications where the risk of pitting is a critical factor, passivation is a common practice to impart greater uniformity to the metal surface.
It is performed by applying oxidizing agents to the surface that dissolve iron, but not oxides of alloying elements. ASTM A967-1 standard suggests using 8% citric acid for 3 hours at room temperature as a simple and relatively safe method. Passivation is faster when using 20% nitric acid for 30 minutes at 55°C. To improve the passivation process, 2% sodium dichromate can also be added to the nitric acid, but this significantly reduces safety. Hydrofluoric acid can also be used to passivate stainless steel, but this process is very dangerous. In the pharmaceutical industry, a particularly pure solution of phosphoric acid is sometimes used for passivation. The acids used for passivation must be virtually free of chloride or fluoride ions, otherwise pitting corrosion of the steel may occur.
The speed of the passivation process using both nitric and citric acid can be increased by increasing the temperature. Passivation can last from several minutes to several days, depending on the grade of steel being processed.
ASTM standards are general guidelines only. Chemicals, conditions and exposure times should be selected in accordance with the expected operating conditions, including the nature of the corrosive environment.
Testing the effectiveness of passivation can be electrochemical, using polarization curves and potential maps, or chemical, by analyzing for copper sulfates or ferrocyanides. Electrochemical methods are more advanced, they improve accuracy and provide more information.
Where the positive portion of the curve is vertical or close to vertical, there is a passive region, i.e. There is a high-strength thin film of chromium on the surface. The voltage range over which the film remains stable is an indicator of its quality.
Sensitization of stainless steels and corrosion of welds
Sensitization of stainless steel is a type of intergranular (intergranular) corrosion that causes steel crystals to fall out from the surface of the metal, as shown in the photo above. When this phenomenon occurs in the weld joint area, it is often referred to as weld corrosion. If sensitization occurs within a narrow band, it is called knife corrosion: in the past, the heated area of a steel knife blade near the handle tended to lose crystals, leaving blackened gouges. 316 stainless steel can be sensitized when heated to temperatures in the range of 480-900°C. At higher temperatures, sensitization may begin after as little as 3 minutes. If the temperature is lower, it will take more than an hour.
Sensitization causes corrosion as the grain boundaries lose chromium due to the formation of intermetallic carbides. Six carbon atoms remove 23 chromium atoms from the alloy. This could result in a reduction in local chromium content from 18 to 12%. When sensitized stainless steel encounters an aggressive environment, the center of the crystal becomes the cathode and the grain boundary becomes a very active local anode. The initial period of development of the process may be delayed, since the destruction of surface crystals takes a long time. However, when the intergranular bonds weaken, the crystals fall out from the surface and leave blackish pits.
Contact corrosion
The passive surface of stainless steel is constantly being transformed. If steel comes into contact with carbon or ferritic steel, particles may remain on the surface and form local anodes. The resulting corrosion is unsightly. Contact between these types of metals should be avoided. It is necessary to use separate tools for different types of materials; work areas should be separated.
Corrosion and surface treatment of stainless steel
There are many ways to treat the surface of stainless steel products. The photo above shows the milled surface. Brushed, sanded and polished surfaces are also common. Typically, the choice of surface treatment for stainless steel is based on the external preferences of the architect or designer, but considerations of corrosion resistance should also be taken into account. In general, the smoother the surface of the steel, the more resistant it is to corrosion and rust stains. Rough surfaces are prone to pitting in conditions where smoother surfaces would be resistant. Rough surfaces accumulate dirt and require more maintenance. Steel grades such as 304 or 316 are only marginally resistant to rust staining when used in marine or food processing applications and are definitely vulnerable if the products have a rough surface.
Care of stainless steel.
If stainless steel is to remain looking good, don't assume it can handle maintenance. In an urban environment or in marine conditions, regular washing with warm water containing surfactants is required to maintain a decent appearance. Usually the interval between cleanings is about six months, but in harsh climates more regular washing may be required. Cleaners containing active ingredients such as chlorides or ammonia should be strictly avoided. If stains or pits are found on the surface of the steel, remove the stains with a hard sponge. Once pitting appears, more regular care will be required. You can find methods for cleaning stainless steel in this article.
Corrosion of stainless steel at construction sites
Stainless steel is often used on the exterior of modern buildings because it is attractive and easy to maintain. Corrosion similar to that shown in the photo above can occur if stainless steel comes into contact with corrosive environments or ferritic steel during the construction process. These types of surface stains can easily occur if the maintenance regime is not followed if the building is located in a coastal (marine) or industrial area. Steels 304 and 316 require regular maintenance in such conditions.
Corrosion of stainless steel kitchen equipment
The photo shows the consequences of non-compliance with the maintenance regime in the kitchen of a catering establishment. Equipment such as stainless steel shelves or countertops are often made from steel grades less than 316, which are easier to form (AISI 304). Commercial refrigerators and dishwashers are almost always made from the more corrosion-resistant 316 or 316L steels.
Stainless steel kitchen surfaces can quickly corrode if the equipment arrives in poor condition.
On the right is an extreme case of pitting: a chlorine-containing cleaner has corroded a sink. Milder forms of this type of corrosion occur when the wrong cleaner or bleach comes into contact with the stainless steel.
Corrosion of stainless steel products in the pharmaceutical industry
Many pharmaceutical factories work with brine solutions and use 316L stainless steel. Stainless steel usually copes well with these conditions, but if the edge joints remain in contact with the brine, crevice corrosion can occur, as shown in the photo.
When sterilizing with steam, the surface of stainless steel may become covered with reddish spots. Stainless steel used in the pharmaceutical industry can also be subject to pitting corrosion if the process fluid is not fluid enough. Non-flowing solutions can also corrode stainless steel ball and butterfly valves. Disinfectant fumes, such as peracetic acid fumes, can also damage stainless steels. Where variable pumps are used, stainless steel may be susceptible to corrosion by stray currents.
Corrosion of stainless steels in the food industry
This photo shows corrosion on a steel dairy sprayer that started on the inside and worked its way out. Dairy and other products often contain salt. If they are in contact with stainless steel for a long time, corrosion may occur.
Food processing conveyors like the one pictured can quickly corrode if the surface condition is poor. The surface of this conveyor was shot blasted. The cleaned surfaces at the same facility remained in good condition. In the meat industry, sterility is critical and the use of chloride-containing cleaners is often necessary. After processing, they must be carefully removed from the surface.
Corrosion of stainless steel in swimming pools
Stainless steel grab bars are often found in swimming pools and are generally resistant to the corrosion that pool chemicals can cause. The corrosion shown in the photo above was caused by the wrong choice of floor cleaner. Stainless steel products specifically designed for use in swimming pools require regular cleaning and washing.
When ordering stainless steel products from, you can be sure that they will be manufactured taking into account operating conditions. This will allow you to minimize the risk of product damage due to corrosion.
We recommend that you read the articles:
Determining the cost of manufacturing metal products
Ways to reduce the cost of manufacturing metal products.
Innovative technologies for welding work
How to protect a structure or assembly from contact corrosion?
If for design reasons it is not possible to avoid unwanted contact of dissimilar metals, then attempts can be made to reduce galvanic corrosion using the following methods:
- painting surfaces in the area of their junction;
- application of compatible metal coatings;
- isolation of the connection from the external environment;
- electrical insulation;
- installation of non-metallic gaskets, inserts, washers in bolted joints.
Practice shows that in cases where the requirements for the admissibility of contacts of different metals are neglected, one has to pay dearly for this. Incorrect arrangement of contact pairs damages fastening units and metal structures and can cost human life.
Useful tips Updated: 09.29.2020 10:26:57