01/12/2022 Author: VT-METALL
From this material you will learn
:
- 3 signs of metal corrosion
- Main types of metal corrosion depending on the type of aggressive environment
- Types of metal corrosion according to the nature of destruction
- Protective properties of metal oxide films
- 5 ways to protect metals from corrosion
There are different types of metal corrosion. They are classified according to the type of exposure to an aggressive environment: atmospheric, underground, electric; and by the nature of the destruction: continuous, selective, local. Each metal and alloy resists the corrosion process differently.
If we talk about natural protection, then this is an oxide film. Various technologies are also used to prevent and prevent corrosion. The simplest and most widespread is coloring. You will learn about the types of metal corrosion and methods of protection from our material.
Main types of metal corrosion depending on the type of aggressive environment
An environment that causes rapid wear of materials and structures is called aggressive. Depending on its type, we will consider what types of metal corrosion there are.
- The destruction of metal under the influence of air or gas under conditions of high humidity is called atmospheric corrosion.
- If there is no moisture, but the reaction occurs with the participation of gases, this type of corrosion is called gas corrosion. It is important to understand that the absence of moisture is not the only characteristic of this type. It can also occur under the influence of elevated temperature. This type of destruction is usually observed in chemical plants or during the processing of petrochemical products.
- Under the influence of radioactive rays, radiation types of corrosion of metals are considered with examples. Metal can be destroyed very quickly by intense radiation.
- Structures and metals located deep in the ground are most often susceptible to the following type of corrosion - underground.
- Another type of destruction - contact corrosion - occurs when different metals interact with different standard potential characteristics.
- Biological corrosion is a type of damage caused by a reaction to waste products of bacteria, microorganisms, and other large living organisms.
- Types of chemical corrosion of metals and alloys that are influenced by current from external sources or currents arising in the conductive environment of the soil are called electrocorrosion. It is also divided into two subtypes: external current corrosion and stray current corrosion.
- The combined type of effect on metals is called cavitation wear. Here, in parallel, the object is affected by corrosion of the external environment and shock.
- The following mixed characteristic of the impact on metals and structures using standard corrosion influence and variable mechanical stresses is called stress corrosion, or corrosion cracking. It occurs in aggressive environments under high loads or due to the sensitivity of alloys.
- The classification of types of metal corrosion based simultaneously on corrosion and vibration effects is called fretting corrosion. The mobility of structures causes friction and vibration, therefore, in order to reduce negative manifestations, you need to carefully select the material of the products or use special coatings.
METHODS OF PROTECTION AGAINST CORROSION, THEIR EFFECTIVENESS
There are numerous ways to protect metal from damage or rust. The choice of one method or another is determined by the specific operating and storage conditions of metal products. The most widely used are: steel alloying, metal coating, electrochemical protection.
Alloying is most effective under conditions of mechanical stress and a corrosive environment. Alloying also helps prevent corrosion cracking of products.
For example, the group of steels with special chemical properties includes corrosion-resistant steels. They are obtained by introducing significant additions of chromium or chromium and nickel into carbon and low-alloy steels. With a chromium content of 13, 17 and 25%, chromium steels are not only corrosion-resistant, but also heat-resistant. Chrome-nickel steels have greater corrosion resistance than chromium steels and are widely used in the chemical industry.
Metal coatings are applied to the surface of a product with a thin layer of metal that is sufficiently resistant in a given environment. This coating also gives the surface layers of metal products the required hardness and wear resistance. There are two types of coatings - anodic and cathodic. For iron-carbon alloys, such an anodic coating can be a coating of zinc and cadmium. In water and in humid air, zinc is covered with a layer of basic white carbon dioxide salt, protecting it from further destruction. Zinc coatings are widely used to protect fittings, pipes and tanks from water and hot liquids.
Metal coatings are applied in various ways. The most commonly used method is hot method, galvanization and metallization.
With the hot method, the product is immersed in molten metal, which wets its surface and covers it with a thin layer. Then the product is removed from the bath and cooled. Using this method, the product is coated with a layer of tin or zinc. Tinning is used in the manufacture of tinplate, in the installation of coatings on the internal surfaces of food boilers and other products. Galvanizing protects, for example, roofing iron and water pipes from corrosion.
In the galvanic method, metal products are placed in a galvanic bath. Under the influence of electric current, cathodic deposition of a film of protective metal occurs on the surface of the product. The thickness of the coating can be adjusted within a wide range. Coatings are also obtained by spraying molten metal using special metallization guns and spraying the protected metal onto its surface. This type of protection is used for large-sized structures: railway bridges, etc. Aluminum, zinc, chromium, and corrosion-resistant steels are used as protective metals.
Non-metallic coatings are made from varnishes, paints, enamels and other substances and isolate the product from the effects of the external environment. They are easily applied to the product, close pores well, do not change the properties of the metal and are relatively cheap. During storage and transportation, metal products are coated with special lubricants, mineral oils and fats. To protect products operating in highly aggressive environments, plastic coatings made of vinyl plastic and polyvinyl chloride are used.
Chemical coatings - protective oxide and other films - are created when metal is exposed to strong chemical reagents. Oxidation and phosphating of metal products are also widely used.
Oxidation is the creation of an oxide film on the surface of a product that is highly resistant to corrosion. It is most widely used for corrosion protection of products made of aluminum and its alloys.
Phosphating of steel products consists of creating a surface layer of manganese and iron phosphates. Phosphate coatings are subsequently used as a sublayer, often in combination with lubricants, to reduce friction when processing metals by pressure, drawing, and for good running-in of rubbing machine parts.
In some cases, they resort to protecting metals from corrosion using protectors. The essence of tread protection is that protectors - pieces of metal - are attached to the surface of the protected product. A galvanic couple is formed in which the anode is the protector and the cathode is the product. As a result, the protector is destroyed, protecting the product. In this way, for example, underwater metal parts of ships are protected by attaching zinc plates to them.
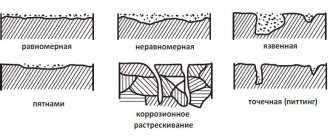
Types of metal corrosion according to the nature of destruction
Several types of corrosion are distinguished by the nature of corrosion lesions. Let's list them:
- Continuous - distributed over the entire plane of the metal. It is uniform if the speed of impact on the entire surface is the same. If the rate of destruction is different, this type will be called uneven corrosion.
- It happens that only one specific component of the alloy or a certain component of it deteriorates. Then we talk about selective corrosion.
- Small dots, cavities, spots, depressions of varying thickness - all these characteristics confirm that the next type of corrosion has come into play - local.
- Partial corrosion that begins first on the surface and then penetrates deeper in some places is called subsurface. These types of chemical corrosion of metals and alloys lead to delamination.
- One of the most dangerous types of corrosion occurs at grain boundaries. In fact, transgranular or intergranular corrosion is difficult to detect by eye. The most affected steel (chromium and chromium-nickel), aluminum and nickel alloys suffer from it. Products under the influence of corrosion become very brittle, and indicators of strength and ductility change significantly.
- Crevice corrosion is a type of local metal corrosion that appears in the spaces between parts, gaps, and fastening joints.
NATIONAL ECONOMIC IMPORTANCE OF COMBATING CORROSION
Losses from corrosion can be divided into direct and indirect. Direct losses are the cost of replaced products, the cost of protective measures and irretrievable loss of metal due to corrosion. According to experts, on a global scale these currently account for about 10...15% of steel production. Indirect – product losses as a result of leaks, decreased unit performance, contamination of the target product with corrosion products, etc.
A significant part of the power of ferrous metallurgy enterprises is spent on replenishing metal losses due to corrosion. However, this does not fully reflect the actual damage associated with the failure of metal products. Significant losses are caused by equipment failures, downtime, losses and waste in metalworking, violations of product quality and, ultimately, an increase in its cost and a decrease in labor productivity. Therefore, saving metal, improving the quality of raw materials and metal products, reducing corrosion losses is an indispensable condition for increasing production efficiency and product quality, which must be ensured on a national scale.
Electrochemical corrosion.
Electrochemical corrosion is the most common type of metal corrosion. By an electrochemical mechanism, metals corrode in contact with electrolyte solutions (sea water, solutions of acids, alkalis, salts). Under normal atmospheric conditions and in the ground, metals also corrode by an electrochemical mechanism, because on their surface there are drops of moisture with dissolved components of air and earth. Electrochemical corrosion is a heterogeneous and multistage process. Its cause is the thermodynamic instability of metals in a given corrosive environment.
Thermodynamics of electrochemical corrosion of metals.
The tendency of metals to transition from a metallic state to an ionic state is different for different metals. The probability of such a transition also depends on the nature of the corrosive environment. This probability can be expressed by a decrease in free energy when a transition reaction occurs in a given environment under certain conditions.
Therefore, for the electrochemical dissolution of a metal, the presence in the solution of an oxidizing agent (a depolarizer that would carry out the cathodic reaction of electron assimilation) is necessary, the reversible redox potential of which is more positive than the reversible potential of the metal under given conditions.
Cathodic processes during electrochemical corrosion can be carried out by various substances.
- ions
- oxides and hydroxides (usually poorly soluble corrosion products formed on the surface of metals)
- organic compounds
molecules
In corrosion practice, hydrogen ions and molecules of oxygen dissolved in the electrolyte act as oxidizing depolarizers that carry out corrosion.
With an increase in the activity of metal ions (increasing the concentration of metal ions in the solution), the anode potential increases, which leads to inhibition of metal dissolution. A decrease in the activity of the metal, on the contrary, promotes the dissolution of the metal. During the corrosion process, not only the properties of the metal surface change, but also the contacting solution (changes in the concentration of its individual components). When, for example, the concentration of the depolarizer decreases, it may turn out at the cathode zone that the cathodic depolarization reaction is thermodynamically impossible.
Homogeneous and heterogeneous paths of electrochemical corrosion.
The reason for the corrosion of metals in solutions that do not contain ions of the same name is explained by the theory of irreversible potentials. This theory considers the surface of metals as uniform, homogeneous. The main and only reason for the dissolution (corrosion) of such metals is the thermodynamic possibility of the occurrence of anodic and cathodic events. The rate of dissolution (corrosion) will be determined by kinetic factors. But the homogeneous surface of metals can be considered as a limiting case, which can be realized, for example, in liquid metals. (mercury and metal amalgams). For solid metals, such an assumption will be erroneous, if only because different atoms of the alloy (and pure metal) occupy different positions in the crystal lattice. The strongest deviation from a homogeneous structure will be observed in the presence of foreign inclusions, intermetallic compounds, grain boundaries, etc. in the metal. In this case, of course, the surface is heterogeneous. It has been established that even if there are inhomogeneities on the metal surface, the surface as a whole remains equipotential.
Thus, the heterogeneity of the alloy surfaces cannot be the main cause of general metal corrosion. The most significant in such cases is the ionization of the dissolution of the anodic component near the cathode component; this is possible if galvanic elements appear on the surface of the metal structure. Let's look at some of them:
a) heterogeneity of the metal phase due to the heterogeneity of the alloy, as well as as a result of micro and macro inclusions.
b) inhomogeneity of the metal surface due to the presence of boundaries of blocks and crystal grains, dislocations reaching the surface, anisotropy of crystals.
c), d) heterogeneity of protective films on the surface due to micro and macropores of the film (c), due to the uneven formation of secondary corrosion products on the surface (d), etc.
We examined two extreme mechanisms of self-dissolution of metals: uniform dissolution of an ideally homogeneous surface and dissolution (mostly local) of microelements with the spatial separation of cathode and anodic zones (processes).
In general, it is necessary to take into account the possibility that cathodic processes occur in the anodic areas along with the main anodic processes, while in the cathodic areas anodic dissolution processes can occur at a reduced rate.
We can conclude that there is no reason to contrast “homogeneous” and “heterogeneous” paths of corrosion processes. It would be more correct to consider them as factors that complement each other. The main cause of corrosion of metals remains the thermodynamic probability of the occurrence of anodic processes of metal ionization and the associated cathodic depolarization process under given conditions on the metal.
Anodic processes during electrochemical corrosion of metals.
Thermodynamic fundamentals.
For the corrosion process to occur, the state of the compound in which the metal cation is found in solution is essential. Ionization of the metal followed by the transition of simple metal components into solution represents only one of the possible directions of anodic processes. The shape of their specific state is largely determined both by the nature of the metal and the medium in contact with it, and by the direction and magnitude of the polarizing current (or electrode potential). When entering the solution, the corroding metal comes into contact with either the solvent or the components of the solution. In this case, simple and complex compounds with different solubility and different adhesion to the metal surface can be formed. At high positive potential values at the anode, the process of water oxidation with the release of oxygen is possible. Depending on what processes or combinations thereof occur at the anode, they can largely (and sometimes completely) control the overall corrosion process.
Reasons for anodic dissolution of metals.
The simplest anodic reactions are those that result in the formation of soluble hydrated and complex cations. which are removed from the anode by diffusion, migration (transfer due to an electric field) or convection.
Polar molecules of a liquid electrostatically interact with charged ions and form solvate (in the case of water, hydrate) complexes. Possessing a significantly lower energy reserve than ions in the metal crystal lattice. The magnitude of this decrease can be estimated based on the considerations proposed by Born. A total electric charge in a vacuum has energy equal to potential energy. To determine the amount of charge energy, imagine that a conducting sphere of radius r has a charge q. The introduction of another part of the charge dq into the sphere must be met by repulsive forces df=qdq/r. A truly enormous decrease in the energy of an ion in an aqueous solution indicates the stability of such a state in it. Thus, the reason for the transfer of metal atoms from the surface and their ionization is the electrostatic interaction (solvation) of metal ions with polar solvent molecules.
Anodic passivity of metals.
With significant inhibition of the anodic reaction of metal ionization, the rate of the corrosion process can decrease by several orders of magnitude. This state of the metal is usually called anodic passivity. Passivity can be defined as follows: passivity is a state of increased corrosion resistance of a metal or alloy (under conditions where it is thermodynamically reactive), caused by preferential inhibition of the anodic process, i.e. It may happen that in real conditions the rate of corrosion of “active” elements turns out to be very insignificant due to the onset of a passive state. For example, titanium, located to the left of zinc, and chromium, located next to zinc, due to the onset of passivity, are more corrosion resistant in most aqueous environments than zinc. The nature of the metal-solution system influences the tendency towards a passive state. Ti, Ni, Al, Mg, Fe, Co, etc. exhibit the greatest tendency to transition to a passive state.
The onset of a passive state leads to a significant change in the shape of the anodic polarization curve. The curve can be divided into several characteristic sections:
But starting from B, the process of formation of a protective layer (phase or adsorption) becomes possible, the rate of which increases as the potential shifts in the positive direction. This leads to inhibition of anodic dissolution (BD). At point D, corresponding to the potential (passivation onset potential), the rate of formation of the protective layer is equal to the rate of its dissolution. Next comes the growth of a protective layer shielding the surface, and the rate of anodic dissolution sharply decreases (DE). At point E, corresponding to the complete passivation potential, the metal is in a passive state. In the EF section (passive state region), the rate of the anodic process does not depend on the potential, but is determined by the rate of chemical dissolution of the protective film. The current corresponding to the region of the passive state is called the passive state current (i). More positive than F, a new branch of active dissolution with the formation of cations of higher valency is possible (-repassivation potential).
At high positive potentials, localized breakdown of the oxide film is possible - the metal begins to dissolve according to the type of pitting (PP') called pitting potential.
A metal passivated in a given environment can remain in a passive state for some time in a non-passivating environment.
Metal coatings
These methods of preventing corrosion involve immersing the steel in a molten metal whose electrical potential is lower than that of iron (the greater the difference, the more effective the coating).
Practical applications include electroplating with zinc or tin, as well as diffusion coatings with nickel, chromium, silicon or aluminum. Compared to other corrosion protection methods, galvanization is known for its lower initial costs, durability and versatility.
Since the consumption of protector metal is quite high, technologies that are characterized by the efficiency of the components used and the strength of the coatings created are advantageous. First on this list is galvanizing. The iron in the steel reacts with the zinc to form a strong alloy coating that serves as protection.
Types of Rust
Iron is more susceptible to corrosion. From a chemical point of view, rust is an oxidative process (like combustion). Elements resulting from oxidation in an oxygen environment are called Oxides. There are 4 main types.
1. Yellow rust - chemical formula FeO(OH)H2O (ferrous oxide). Occurs in a humid, oxygen-poor environment. Often found underwater. In nature, it exists in the form of the mineral wustite, while being a monoxide (it contains 1 oxygen atom).
2. Brown rust - Fe2O3 (double iron oxide): grows without water and is rare.
3. Black rust - Fe3O4 (tetravalent iron oxide). Formed with low oxygen content and without water, it is therefore stable and spreads very slowly. This oxide is ferromagnetic (under certain conditions it is magnetized in the absence of an external magnetic field), therefore it is potentially applicable for the creation of superconductors.
4. Red rust - chemical formula Fe2O3•H2O (ferric oxide). Occurs under the influence of oxygen and water, the most common type, the process proceeds evenly and affects the entire surface. Unlike all of the above types of oxidation, which are not so dangerous for iron, this one forms iron hydroxide in its thickness, which, when it begins to peel off, opens up more and more layers of metal for destruction. The reaction can continue until the structure is completely destroyed. It is used in iron smelting and as a dye in the food industry. It occurs naturally in nature under the name hematid.
Several types of rusting can occur simultaneously without particularly interfering with each other.
Chemical and electrochemical corrosion
Iron rusts if it contains additives and impurities (such as carbon) and comes into contact with water and oxygen. If salt (sodium and potassium chloride) is dissolved in water, the reaction becomes electrochemical and the rusting process accelerates. The massive use of these salts both in household chemicals and for combating ice and snow makes electrochemical corrosion a very common and dangerous phenomenon: losses in the United States from the use of salts in winter amount to $2.5 billion. When exposed to water and oxygen simultaneously, iron hydroxide is formed, which, unlike oxide, peels off from the metal and does not protect it in any way. The reaction continues either until the iron is completely destroyed or until the system runs out of water or oxygen.
Electrochemical corrosion can be caused by stray currents that occur when part of the current leaks from an electrical circuit into aqueous solutions or into the soil and from there into a metal structure. In those places where stray currents exit metal structures back into water or soil, metal destruction occurs. Stray currents occur especially often in places where ground electric transport moves (for example, trams and electric railway locomotives). In just one year, stray currents with a force of 1A are capable of dissolving 9.1 kg of iron, 10.7 kg of zinc, and 33.4 kg of lead.
In the second part of the article we will tell you how you can protect your metal structures from this scourge or defeat it if it is already attacking.
Protective paints for metal
Applying special protective paints to a metal surface is one of the most effective means against corrosion. When dry, they form a hard film containing pigments. The thickness of this film may vary depending on the purpose of the metal product. The thickness and nature of the interaction of the paint with the surface determine the protective properties of the coating.
Anti-corrosion agents for metal can be divided into three groups:
- primers;
- paints;
- products for applying directly over rust.
When choosing a protective paint, it is important to consider the properties of the metal surface on which it will be applied. For example, for ferrous metals such as steel, it is better to choose a primer that contains zinc. The fact is that a galvanized surface can withstand destruction for a long time. As a rule, the instructions contain information about what type of surface the product is intended for.
Rust paint becomes a good solution in a situation where the surface cannot be properly cleaned of rust. It is simple and easy to use, lays down in an even dense layer. The coating created by this paint is durable and resistant to corrosion. Despite the fact that there are already pockets of corrosion on the metal surface, rust paint will not allow them to grow and spread.
Most products are suitable for manual application at home. Some paints apply better if you spray them. The composition of the paints takes into account the fact that they will be used, among other things, to protect structures located on the street. The products can be applied outdoors. As a rule, anti-corrosion paints are applied in a fairly thick layer for best effect.
The painted surface looks aesthetically pleasing. At the same time, it is reliably protected from corrosion. The film formed as a result of dyeing prevents the negative effects of light, moisture, and impurities in the atmosphere. Surface protection against oxidation is provided for up to 8 years.