Corrosion (from Late Latin corrōsiōn “to gnaw, chew”) is a gradually developing process of surface damage to metals that have the ability to actively react with oxygen. One clear example of this phenomenon is metal corrosion due to the formation of iron oxide Fe2O3 or rust. It is characteristic that the other two iron oxides - oxide FeO and oxide-oxide Fe3O4 - have a significantly lower corrosive ability, and Fe3O4 under some conditions can even play the role of a solid lubricant.
Corrosion theory suggests that for a metal to begin to deteriorate, four main components must be present:
- Cathode.
- Anode.
- The electrical connection that exists between the cathode and the anode.
- Electrolyte or any other conductive medium that facilitates the directed movement of ions.
The normal formation of rust on steel can be thought of as an electric battery. When metal atoms are exposed to an environment that contains oxygen, the metal produces electrons. This action can be locally limited to create a crack or micro-indentation. As the process progresses, corrosion spreads to the surrounding area, which will lead to a general deterioration of the surface condition. Limited (pitting) corrosion can cause metal fatigue - a decrease in its strength characteristics, and existing corrosive agents, for example, sea water, can lead to progressive crack growth.
The theory of corrosion also states that the intensification of surface destruction of metal is facilitated by microstructural changes that occur at elevated temperatures, in particular during welding. This is explained by increased values of activation energy, due to which the number of ions conducting electric current increases exponentially.
Definition of corrosion
Corrosion is the gradual destruction of objects, usually metals, caused by an active electrolyte environment and a chemical oxidation reaction.
The essence of the corrosion process is the presence of a constantly operating anodic reaction. It is caused by the dissolution of a metal, which generates electrons. Part of the activation energy is additionally spent on another process called the cathodic reaction. These two processes balance the charges produced. The zones causing these processes can be located close or far from each other, depending on the situation.
The electrons generated in the process must be consumed through a cathodic reaction. Hydrogen ions and electrons react to form atomic and then hydrogen gas. However, hydrogen is a powerful reducing agent, so further corrosion can be prevented by creating a thin film of gas on the metal surface. It serves as a polarizer, reducing metal contact with water and reducing corrosion. Thus, anything that destroys the barrier film increases the rate of corrosion.
The main factors determining the intensity of the process are:
- Speed;
- Temperature;
- The level of mechanical and thermal stresses that arise;
- The nature of the ongoing chemical reactions.
Corrosion hinders the introduction of new metal materials into production and causes significant damage to the economy.
Application of corrosion inhibitors
Based on their properties, these complex chemical compositions are divided into two groups - agents that reduce the corrosive activity of the external environment (passivators) and complexes that form an adsorbent-type layer on the surface of the protected product that protects against corrosion (adsorbators).
By the way, for those who do not know exactly how asorption and adsorption differ. In the first case, this is the absorption of substances from a gaseous medium by a liquid, in the second, the absorption of substances from the same medium by the surface layer of a solid.
Very often, inhibitors are used in mains and local heating systems. In this case, internal corrosion of pipelines and heating radiators under the influence of the coolant is significantly reduced.
Important: in autonomous closed systems operating on distilled water, the use of corrosion inhibitors is usually not justified.
Also, inhibitors, in combination with other components, are used for the prevention and cleaning of water pipes and water heating devices.
The use of inhibitors in construction is also widespread, including in the creation of reinforcement for reinforced concrete structures. Treatment with a special compound prevents corrosion of the reinforcement in the monolith and extends the service life of reinforced concrete. The load capacity of the structure is also preserved, since the integrity of the reinforcement allows maintaining a normal level of load resistance.
Inhibitors are used both in the form of liquids and in the form of gases introduced into the environment surrounding the product.
Types of metal corrosion
All corrosion phenomena can be classified according to the following parameters:
- According to the degree of uniformity. A distinction is made between surface corrosion, which uniformly reduces the thickness of the surface, and uneven corrosion - pitting or pitting;
- According to the intensity of impact on the metal. For example, selective corrosion destroys only certain structural components, while contact corrosion affects the less corrosion-resistant (“base”) components of friction pairs;
- With intergranular corrosion, destruction occurs along the grain boundaries and spreads deep into the metal.
- Fretting corrosion, when two bodies in contact with each other perform oscillatory movements of small amplitude (no more than 100 microns) relative to each other.
When exposed to tensile stresses and an aggressive environment at the same time, corrosion cracking of an intergranular or transgranular nature is observed, and when alternating stresses are applied, corrosion-fatigue failure begins. Protecting metals from corrosion thus also involves simultaneously reducing wear of parts.
Next, we discuss the main phenomena that occur in damaged areas during various types of corrosion.
Lecture on the topic: “Corrosion and protection of metals”
Topic 5. Corrosion and protection of metals
5.1. Definition and classification of corrosion processes
Corrosion
– this is the destruction of a metal as a result of its physical and chemical interaction with the environment. In this case, metals are oxidized and products are formed, the composition of which depends on the corrosion conditions.
The chemical energy of the reaction of corrosive destruction of metals is released in the form of heat and dissipated in the surrounding space.
Corrosion causes large losses. Irreversible losses of metals from corrosion amount to 15% of their annual production.
However, in many cases, indirect losses from corrosion can significantly exceed direct losses due to metal dissolution. Replacing a corroded boiler or condenser at a large thermal power plant can cause significant damage to the power system. In addition, losses from corrosion can also include the cost of lost product, such as oil, gas, water, through a system with corroded pipes or antifreeze through a corroded radiator.
In general, losses to the national economy from corrosion amount to many billions of rubles annually. The study of corrosion and the development of methods for protecting metals from it are of theoretical interest and are of great economic importance. According to the mechanism of the corrosion process, depending on the nature of the external environment with which the metal interacts, a distinction is made between chemical and electrochemical corrosion.
The environment in which metal corrodes (corrodes) is called a corrosive or aggressive environment.
In the case of metals, when talking about their corrosion, they mean the undesirable process of interaction of the metal with the environment. The physical and chemical essence of the changes that metal undergoes during corrosion is metal oxidation.
Any corrosion process is multi-stage:
1) It is necessary to supply the corrosive medium or its individual components to the metal surface.
2) Interaction of the environment with the metal.
3) Complete or partial removal of products from the metal surface (into the volume of liquid, if the medium is liquid).
It is known that most metals (except Ag, Pt, Cu, Au) are found in nature in the ionic state: oxides, sulfides, carbonates, etc., usually called metal ores.
The ionic state is more favorable; it is characterized by lower internal energy. This is noticeable when obtaining metals from ores and their corrosion. The absorbed energy during the reduction of metal from compounds indicates that the free metal has higher energy than the metal compound. This leads to the fact that the metal in contact with a corrosive environment tends to move into an energetically favorable state with a lower energy reserve.
That is, we can say that the root cause of metal corrosion is the thermodynamic instability of metals in a given environment.
Classification of corrosion processes.
1. According to the mechanism of the process, chemical and electrochemical corrosion of metal is distinguished.
Chemical corrosion is the interaction of metals with a corrosive environment, in which the metal is oxidized and the oxidizing components of the corrosive environment are reduced in one act. This is how the oxidation of most metals occurs in gas environments containing an oxidizing agent (for example, oxidation in air with increasing temperature)
Mg+ O -> MgO
4Al + 3O -> 2AlO
Electrochemical corrosion is the interaction of a metal with a corrosive environment, in which the ionization of metal atoms and the reduction of the oxidizing component of the environment occurs not in water, and their rates depend on the electrode potential of the metal. This process involves, for example, the interaction of a metal with acids:
Zn + 2HCl -> Zn +2Cl +H
this total reaction consists of two acts:
Zn -> Zn + 2e
2H + 2e -> H
2. By the nature of corrosion destruction.
General or complete corrosion in which the entire surface of the metal is corroded. It is accordingly divided into uniform (1a), non-uniform (1b) and selective (1c), in which the corrosion process spreads predominantly over any structural component of the alloy.
Local corrosion in which certain areas of the metal corrode:
a) corrosion by pitting - corrosion damage in the form of separate medium and large spots (corrosion of brass in sea water)
b) intercrystalline corrosion, in which the corrosion process spreads along the metal-alloy boundary (aluminum is alloyed with chromium-nickel) and other types of corrosion.
3. According to the conditions of the process.
a) Gas corrosion is corrosion in a gas environment at high temperatures. (liquid metal, during hot rolling, stamping, etc.)
b) Atmospheric corrosion is the corrosion of metal in the natural atmosphere or the atmosphere of a workshop (roof rusting, aircraft skin corrosion).
c) Liquid corrosion is corrosion in liquid media: both in electrolyte solutions and in non-electrolyte solutions.
d) Underground corrosion is the corrosion of metal in the soil
e) Structural corrosion - corrosion due to the structural heterogeneity of the metal.
f) Microbiological corrosion - the result of the action of bacteria
g) Corrosion by external current - exposure to an external current source (anodic or cathodic grounding)
h) Corrosion by stray currents - the passage of current along unforeseen paths according to the design.
i) Contact corrosion - the conjugation of dissimilar electrochemical metals in an electrically conductive environment.
j) Stress corrosion - simultaneous exposure to a corrosive environment and mechanical stress.
The rate of corrosion is expressed in several ways. The most commonly used are mass and depth indicators of corrosion. The first of them gives the mass loss (in grams or kilograms) per unit of time (second, hour, day, year) per unit area (square meter) of the test sample.
The depth of corrosion is expressed by a decrease in metal thickness per unit time.
5.2. Chemical corrosion
Of greatest practical importance is chemical corrosion in an environment of hot gases, which is called gas corrosion.
During the oxidation process, a solid film of oxides forms on the metal surface. For corrosion to continue, it is necessary for metal ions or oxygen (or both) to diffuse through this film. Typically, diffusion of metal ions rather than atoms occurs from the metal-oxide interface in the direction from the metal to the outer surface of the film, since metal ions are smaller in size than atoms.
At the same time, electrons must move in the same direction. O2– ions have a larger radius than atoms, therefore, it is not ions that move from the oxide–gas interface into the depth of the film, but oxygen atoms, which are ionized in the film (O + 2e– = O2–) and, meeting metal ions, form oxides .
The protective properties of films depend on the ratio between the volumes of corrosion products Vok and the metal Vme from which they were formed:
If Vok/Vme1, then the resulting film cannot be continuous and protect the metal from corrosion. The rate of film growth over time for such metals remains constant.
For metals in which continuous films (Vok/Vme1) are obtained as a result of chemical corrosion, the corrosion process will be inhibited by the diffusion of reagents through the film, and as the film thickens, its further growth will slow down all the time.
In this case, the film must have a certain optimal thickness in order to sufficiently inhibit the counter-diffusion of molecules of the aggressive agent and metal ions.
The corrosion rate increases with increasing temperature due to an increase in the diffusion coefficient and a change in the protective properties of the film. The rapid destruction of the protective film is often caused by sudden temperature changes. This is primarily due to different coefficients of linear expansion of the metal and film.
Until now, we have considered the formation, stability and destruction of protective oxide films that appear on the metal during its chemical interaction with oxygen. But in addition to oxygen, a number of other gases can have strong aggressive properties towards metals at elevated temperatures.
The most active gases are fluorine, sulfur dioxide, chlorine, and hydrogen sulfide. Their aggressiveness towards various metals, and therefore the rate of corrosion of the latter, is not the same.
For example, aluminum and its alloys, chromium and steels with a high chromium content are stable in an atmosphere containing oxygen as the main aggressive agent, but become completely unstable if chlorine is present in the atmosphere. Nickel is unstable in an atmosphere of sulfur dioxide, but copper is quite stable.
Corrosion of low-alloy and carbon steels in the exhaust gases of internal combustion engines, in flue and furnace gases strongly depends on the ratio of CO and O2. An increase in O2 content increases the rate of gas corrosion and, conversely, an increase in CO content weakens corrosion. A number of metals (CO, Ni, Cu, Pb, Cd, Ti) are stable in an atmosphere of pure water vapor at temperatures above the boiling point of water.
5.3. Electrochemical corrosion
Electrochemical corrosion occurs when the salt of a non-uniform metal or alloy is in an electrolyte, that is, in a solution containing ions of various substances. Electrolytes are usually aqueous solutions of salts, acids or alkalis.
It was found that upon contact between metal and electrolyte, a large number of galvanic microelements begin to work, in which the transfer of electrical charges occurs in the same way as in conventional galvanic cells, where one of the electrodes (anode) is destroyed, therefore such corrosion is called electrochemical.
Let us consider in detail the mechanism of electrochemical corrosion.
If you first separately immerse any metal in an electrolyte, then practically there can be two cases: either some of the positively charged metal ions (cations) will go into the solution and the metal will become negatively charged, or some of the positive ions will precipitate from the solution on the metal and charge it positively.
The electrolyte is charged with electricity of the opposite sign, i.e. in the first case it is positive, and in the second case it is negative. In both cases, the metal acquires a certain electrical charge, expressed quantitatively by its electrode potential.
The value of the potential is determined by the ratio of those forces that, on the one hand, hold the ions in the crystal lattice of the metal, and on the other, in the electrolyte. Thus, the electrode potential depends on the nature of the metal, the composition of the electrolyte, as well as on temperature, since the latter affects the magnitude of these forces.
We already know that for a selected electrolyte, all metals can be arranged in the order of changes in their potentials.
Let us now consider what happens if two metals, for example copper and zinc, which have different potentials, are immersed in a common electrolyte.
In this case, the anode will be zinc and the cathode will be copper. A large number of metal ions [Zn++] will pass from the anode into the solution; accordingly, a large excess of electrons will accumulate on the anode.
If we now connect the electrodes to each other with an external conductor, then in the presence of an external conductor, electrons will move along it from the anode to the cathode, i.e., an electric current will flow through the conductor.
Flowing to the cathode (copper), electrons will begin to change its potential in the negative direction; this phenomenon represents a special case of polarization
.
In general, polarization
is called any change in the potential of a working electrode.
On the contrary, restoration of the original potential value is called depolarization
, and substances that contribute to this are called depolarizers.
In this case, such a depolarizer is oxygen, which enters the electrolyte from the air through dissolution.
This oxygen, with the participation of water, can accept electrons that fall on the cathode; in this case, hydroxyl ions [OH–] are formed;
O + H2O + 2e 2OH–
Thus, as a result of depolarization, on the one hand, electrons are removed from the cathode, and on the other hand, hydroxyl groups appear near it. Both of these processes are important. The removal of electrons from the cathode makes it possible for new electrons to flow to it from the anode, and this leads to a further transition of the anode ions into the solution, i.e. to destruction (corrosion) of zinc. And the hydroxyl ions formed at the cathode, moving in the solution by diffusion, meet with zinc ions and form a corrosion product – zinc oxide hydrate:
Zn++ + 2OH–Zn (OH)2
The case considered is an example of oxygen dipolarization corrosion; According to this scheme, steels and aluminum alloys corrode in normal operating environments.
Under certain conditions, processes at the cathode proceed differently. The electrons flowing to the cathode combine with hydrogen ions [H+], which are always found in aqueous solutions, and neutral hydrogen atoms are formed, which, combining in pairs, form hydrogen molecules, which are released from the cathode in the form of a gas:
2Н + 2е Н2
This case represents corrosion with hydrogen depolarization. According to this corrosion pattern, accompanied by the release of hydrogen, magnesium alloys are destroyed, for example, under the influence of sea water, and this also includes cases of metal dissolution in acids.
As can be seen, the processes of electrochemical corrosion are similar to the processes occurring in galvanic cells.
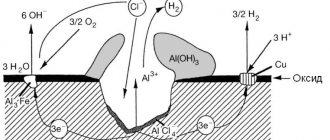
The main difference between the processes of electrochemical corrosion and the processes in a galvanic cell is the absence of an external circuit. During the corrosion process, electrons do not leave the corroding metal, but move inside the metal. The chemical energy of the metal oxidation reaction is transferred not in the form of work, but only in the form of heat. Scheme of electrochemical corrosion of iron in contact with carbon. Iron oxidation reaction occurs at the anodic sites
Fe – 2eFe2+
Hydrogen reduction occurs at the cathode sites
2Н+ + 2е– Н2
The figure shows a diagram of the corrosion of a metal with an energetically inhomogeneous surface containing areas of iron and carbon.
Therefore, the cathodic process will mainly occur in these areas; these are called cathode sites. The presence of areas where cathodic reactions occur faster increases the rate of the corrosion process. In other areas, mainly dissolution of the metal will occur and therefore they are called anodic areas.
The cathode and anodic sections alternate and have very small sizes, i.e. we are talking about microanodes and microcathodes and, accordingly, about corrosive microelements.
Thus, in the presence of energy heterogeneity of the metal surface, the corrosion process consists of the work of the number of corrosion elements. The corrosion element, unlike the galvanic element, is a short-circuited microelement.
Factors affecting corrosion
Factors influencing corrosion are divided into internal, depending on the composition and structure of the alloys present, and external, determined by the nature of the operating environment.
Internal factors.
Alloy composition - some metals and alloys based on them have significant corrosion resistance. These are primarily metals that have positive electrode potentials: copper, gold, platinum, etc. In addition, these include metals that, when oxidized, are capable of forming good protective oxide films, for example: chromium, aluminum and aluminum alloys, copper alloys.
The most resistant are alloys that have a homogeneous structure, because in this case, the grains of the metal or alloy have the same potentials and electrochemical corrosion will not be able to develop.
Particularly unfavorable are cases when the anodic areas are located along the grain boundaries of the alloy, since in this case very dangerous intergranular corrosion develops.
Internal causes of corrosion also include internal stresses that arise during heat treatment or during operation, since the stressed metal has a more negative potential.
External factors
.
These include the composition of the corrosive environment and corrosion conditions: temperature, pressure, speed of movement of the medium. The environment and conditions in which aircraft are operated are very unfavorable, because... Airplanes are outdoors all year round, exposed to precipitation, dust, and sunlight.
The most important factor promoting corrosion is moisture.
1. Typically, atmospheric moisture contains small impurities of salts, acids, and nitrogen oxides formed during lightning discharges. It is often contaminated with industrial gases - sulfur dioxide (SO2), hydrogen sulfide (H2S), hydrogen chloride (HCl), ammonia (NH3), etc. All these substances, dissolving in water, form electrolytes; i.e. an active corrosive environment.
The humid air of coastal areas always contains a lot of salts that come from sea water.
2. Another natural factor that causes corrosion is dust. Even in the absence of visible moisture, dust settled on the metal can be a source of corrosion.
The fact is that dust usually contains various salts that are hygroscopic and can absorb atmospheric moisture. As a result, a corrosion center is created at the site of the settled dust, causing local corrosion of the metal; The corrosion products thus formed are also mostly hygroscopic.
3. Corrosion can be caused by various liquids used in operation (coolants, hydraulic mixtures, acids, etc.).
4. The cause of corrosion is the contact of dissimilar materials in the structure. For example, in contact with copper alloys, steel, aluminum and magnesium alloys are destroyed; the latter are destroyed in contact with steel.
Knowing the potentials of individual alloys in a given operating environment, it is possible to predict in advance the course of the corrosion process and select the correct method of protection.
5.4. Protection of metals from corrosion
Corrosion of metals can be inhibited by changing the potential of the metal, passivating the metal, reducing the concentration of the oxidizing agent, changing the composition of the metal, etc. When developing methods of protection against corrosion, the indicated methods of reducing corrosion and the conditions for its occurrence are used. The choice of method is determined by its effectiveness, as well as economic feasibility. All protection methods are conditionally divided into the following groups:
a) alloying of metals; b) protective coatings (metallic, non-metallic); c) electrochemical protection; d) change in the properties of the corrosive environment; e) rational design of products.
Metal alloying
Metal alloying is an effective (albeit expensive) method of increasing the corrosion resistance of metals. When alloying, components are introduced into the composition that cause passivation of the metal. Passivity
metal is the state of its increased corrosion resistance caused by inhibition of the anodic process. Passivity is mainly caused by the formation of oxide or other protective layers on the metal surface. Chromium, nickel, tungsten, etc. are used as such passivating components.
Protective coatings
Layers artificially created on the surface of metal products and structures to protect them from corrosion are called protective coatings. If, in addition to corrosion protection, the coating also serves for decorative purposes, it is called protective-decorative. The choice of coating type depends on the conditions in which the metal is used. Materials for metal protective coatings can be either pure metals (zinc, cadmium, aluminum, nickel, copper, chromium, silver, etc.) or their alloys (bronze, brass, etc.). Based on the nature of the behavior of metal coatings during corrosion, they can be divided into cathodic and anodic.
Cathodic coatings include coatings whose potentials in a given environment have a more positive value than the potential of the base metal. Examples of cathode coatings on steel include Cu, Ni, Ag.
When the coating is damaged (or the presence of pores), a corrosion element appears in which the main material in the pore serves as an anode and dissolves, and the coating material serves as a cathode, at which hydrogen is released or oxygen is absorbed.
- solution; 2 - coating; 3 - base metal; 4th time)
Consequently, cathodic coatings can protect metal from corrosion only in the absence of pores and damage to the coating.
Anodic coatings have a more negative potential of the base metal. An example of an anodic coating is zinc on steel. In this case, the base metal will be the cathode of the corrosive element, so it will not corrode.
(1- solution; 2- coating; 3- base metal; 4- pore)
To obtain metal protective coatings, various methods are used: electrochemical (electroplating), immersion in molten metal, metallization, thermal diffusion and chemical.
With thermodiffusion
In the coating method, the product is placed in a mixture containing coating metal powder. At elevated temperatures, the applied metal diffuses into the base metal.
The thermal diffusion method is widely used to produce heat-resistant coatings with aluminum (alitizing), silicon (siliconizing), chromium (chrome plating), and titanium (titanizing). Heat-resistant coatings allow you to combine the high heat resistance of the base material with the high heat resistance of the surface layer.
Chemical
The method for producing metal coatings involves the reduction of metal compounds using hydrogen hydrazine (a product of the partial oxidation of ammonia N2H4) and other reducing agents.
Non-metallic protective coatings can be either inorganic or organic. The protective effect of these coatings is mainly reduced to isolating the metal from the environment. Inorganic coatings include inorganic enamels, metal oxides, compounds of chromium, phosphorus, etc. Organic coatings include paint and varnish coatings, coatings with resins, plastics, polymer films, and rubber.
A number of coatings obtained by chemical processing of metal include protective coatings formed directly on the surface of the metal. The formation of protective oxide films on the surface of metal products is called oxidation in technology. Some processes have special names. For example, the processes of applying oxide films to steel are sometimes called bluing
, and electrochemical oxidation of aluminum -
anodization
. The protective properties of oxide films are increased by impregnating them with oil.
Phosphate coatings on steel are obtained from solutions of orthophosphoric acid and manganese or zinc orthophosphates (for example, ZnHPO4 +H3PO4). The reaction produces a porous crystalline metal phosphate that is well bonded to the steel surface. Phosphate coatings by themselves do not provide sufficient corrosion protection. They are used mainly as a substrate for paint, which increases the adhesion of the paintwork to steel and reduces corrosion in scratch areas. The protective properties of a phosphate film obtained on metal are significantly increased after it is coated (or impregnated) with varnish, oil, or wax.
Paint and varnish coatings are the most common and irreplaceable. The paint coating must be continuous, non-porous, gas and waterproof, chemically resistant, elastic, have high adhesion to the material, mechanical strength and hardness. Some coatings have special requirements: increased resistance at high temperatures, resistance to acids, alkalis, gasoline, etc.
Electrochemical protection
This method of protection is based on inhibition of anodic or cathodic reactions of the corrosion process. Electrochemical protection is carried out by attaching a metal with a more negative value of the electrode potential—the protector—to the protected structure, as well as cathodic or anodic polarization due to an externally applied current. Electrochemical protection is most applicable in corrosive environments with good ionic electrical conductivity.
Cathodic polarization is used to protect underground pipelines and cables from corrosion. Cathodic protection is also applied to sluice gates, submarines, water tanks, offshore pipelines and chemical plant equipment.
The essence of cathodic protection is that the protected product is connected to the negative pole of an external direct current source, so it becomes the cathode, and an auxiliary, usually steel, electrode serves as the anode. The auxiliary electrode (anode) dissolves, and hydrogen is released at the protected structure (cathode). If the auxiliary anode is made of a metal that has a more negative potential than the metal being protected, then a galvanic cell occurs. In this case, there is no need to apply current from an external source. The anode dissolves at a rate sufficient to create the necessary electrical current in the system.
Such auxiliary electrodes are called protectors
. For their manufacture, magnesium and its alloys, zinc, and aluminum are mostly used.
Changes in the properties of a corrosive environment
To reduce the aggressiveness of the environment, reduce the concentration of components that are dangerous in terms of corrosion. For example, in neutral environments, corrosion usually occurs with the absorption of oxygen. It is removed by deaeration (boiling, bubbling inert gas) or reduced using appropriate reducing agents (sulfates, hydrazine, etc.). the aggressiveness of the environment can also decrease with a decrease in the concentration of H+ ions, i.e., an increase in pH (alkalinization). Inhibitors are widely used to protect against corrosion.
Inhibitor
is a substance that, when added to the environment where the metal is located, significantly reduces the rate of corrosion of the metal. Inhibitors are used mainly in systems that operate with a constant or little-renewed volume of solution, for example in some chemical apparatus, cooling systems, steam generators, etc. Retarders are especially widely used in metal etching processes to remove scale or rust from the surface.
The mechanism of action of a significant number of inhibitors is the adsorption of the inhibitor on the corroding surface and subsequent inhibition of cathodic or anodic processes.
Cathode retarders reduce the corrosion rate by reducing the intensity of the cathodic process or reducing the area of the cathode sections. Cathodic inhibitors include organic substances containing nitrogen, sulfur and oxygen, for example diethylamine, methenamine, formaldehyde, thiocresol.
In recent years, volatile vapor-phase inhibitors have been widely used. They are used to protect machines, apparatus and other metal products during their operation in an air atmosphere, during transportation and storage. Volatile inhibitors are introduced into containers, packaging materials or placed in close proximity to the working apparatus.
Rational product design
Rational design of products should eliminate the presence or reduce the number and size of particularly dangerous areas from the point of view of corrosion in products or structures (welds, narrow slots, contacts of metals with dissimilar electrode potentials, etc.), and also provide for special protection of the metal of these areas from corrosion .
13
Chemical
Chemical corrosion refers to the gradual destruction of a metal surface due to the reaction of the surface with substances in the external environment. It occurs as a result of metal oxidation with acids to form oxides.
The high-temperature option involves exposing the metal to dry gases. All metals in dry air are covered with a very thin (2...10 microns) layer of oxides. This layer is formed at very high temperatures, when the reaction with oxygen in the air occurs without any restrictions. At room temperature the reaction stops because the oxide film becomes too thin. In the case of, for example, aluminum, such a film consisting of Al2O3 oxide effectively protects the surface of aluminum cookware, since the corrosion resistance of pure aluminum is low.
Chemical corrosion begins at a place where the metal is under pressure and isolated from air circulation. This encourages metal ions to dissolve in a humid environment, which ultimately speeds up the reaction between them and water. The reaction produces hydrous oxides (known as rust when reacting with iron) and free ions.
Kinds
Corrosion processes are classified depending on different criteria. The main ones are color, mechanism of rust formation, type of aggressive environment, nature of destruction.
By color
Depending on the color, there are different types of rust. It can be black, yellow, brown, red. The shade depends on the chemical formula of the resulting substance.
Rusty metal
Yellow
The chemical formula of yellow rust is FeO(OH)H2O. It appears under the influence of high humidity, in an environment with a small amount of oxygen. This type of rust can be seen underwater.
Brown
The chemical formula of brown rust is Fe2O3. It is extremely rare and appears without exposure to moisture.
Red
The chemical formula of red rust is Fe2O3•H2O. Formed by simultaneous exposure to water and oxygen. It is more common than other species. The destructive process proceeds evenly, gradually spreading over the entire surface.
Black
Chemical formula: Fe3O4. Appears without exposure to moisture, in an environment with a small amount of oxygen. Often used to create superconductors because it is ferromagnetic.
According to the flow mechanism
Kinds:
- chemical;
- electromechanical.
The processes differ in the mechanism of material destruction.
Chemical
The process of metal destruction, provoking the disintegration of metal bonds, the development of chemical reactions between the atoms of the material. Elements that interact with each other are not spatially separated. The rate of destruction of a part depends on the speed of the chemical reaction.
Electrochemical
This process of destruction of metal parts occurs in an electrolyte environment and is combined with the generation of current.
Rusty ship
By type of aggressive environment
Kinds:
- Atmospheric.
- Gas.
- Radiation.
- Underground.
- Contact.
- Biocorrosion.
- Electrical corrosion.
- Corrosive cavitation.
- Stress corrosion.
- Fretting corrosion.
Atmospheric
Natural process of destruction. Can occur in air or gas atmosphere. An important condition is a high level of humidity. The higher it is, the faster the material will collapse.
Gas
The process of destruction of metal parts, which occurs in a gas environment. Features low humidity levels. The process of rust formation accelerates as the temperature rises.
Radiation
Occurs under intense exposure to radiation. In high-density alloys it occurs slowly.
Underground
If a metal part lies underground for some time, you may notice a green coating or other color distortions on its surfaces. This is a consequence of oxidative processes that occur in different types of soil.
Contact
Appears quickly in places where two different metals come into contact with each other. This is due to the difference in stationary potential in the electrolyte.
Biocorrosion
The process of destruction of metal parts, which is caused by the influence of various microorganisms and their metabolic products.
Rusty shipwrecks
Corrosion by electric shock
Can occur when exposed to stray or external current. The rate at which rust spreads depends on the current strength, duration, and frequency of its impact on metal parts.
Corrosive cavitation
One of the many processes of self-destruction of different types of metals. It starts when exposed to the external environment, mechanical damage.
Stress Corrosion
The process of destruction of alloys, which occurs when mechanical stress interacts with a corrosive environment. This type of corrosion is dangerous for metal structures that are subject to heavy loads.
Fretting corrosion
A complex corrosion process that occurs under the influence of a corrosive environment with various vibrations. To prevent the formation of rust, it is important to reduce the coefficient of friction of metal parts.
By nature of destruction
Kinds:
- solid;
- selective;
- local;
- subsurface;
- intercrystalline;
- slotted.
They differ in location, degree of penetration into the material, and severity of destruction.
Solid
With this corrosion process, all metal surfaces become covered with rust. It can be uniform or uneven, depending on the rate of destruction of the material in different places of the part.
Electoral
A similar process affects one of the elements of the metal structure, which does not have an anti-corrosion coating that slows down the destruction process.
Rusty car (Photo: pixabay.com)
Local
Rust spots are scattered on the metal surface. They are recesses of different sizes, some of which may be superficial, others through.
Subsurface
Appears under metal surfaces. It quickly penetrates deep into the material. This type of corrosion process is characterized by metal delamination.
Intercrystalline
Begins to appear along the boundaries of individual grains of the material. It is extremely difficult to identify by appearance. Indicators of density, strength, and ductility quickly deteriorate. Parts become fragile.
Slotted
Formed at the junction of two metal parts. May appear in technological gaps, under technical gaskets.
Electrochemical
To simulate the process, it is necessary to consider an iron plate coated with any electrically conductive coating, for example, oxide scale, which was formed during high-temperature processing. When the plate is immersed in a solution of sodium chloride, it is discovered that if the integrity of the scale is damaged, the rusting of the iron will occur much faster in this place. Electrochemical corrosion most reliably explains the rusting of iron under aerobic conditions.
The theory of electrochemical corrosion assumes the presence of additional chemical reactions:
- Fe → Fe ++ + 2e−, - anodic reaction;
- 2e− + O + H2O → 2OH− - cathodic reaction.
When metal ions dissolve, their charge is balanced by chloride ions, which migrate into the area of attack, attracted by the resulting positive ions. Ferric chloride dissolves in water, but this does not prevent further corrosion, since the ferric chloride solution is very acidic due to hydrolysis. As Fe++ ions are removed from this site, they encounter hydroxyl ions, which are either naturally present in the water or formed through a cathodic reaction. The result is the formation and precipitation of iron hydroxide Fe (OH)2. Further, in the presence of dissolved oxygen, it is quickly oxidized to iron oxyhydroxide FeOOH.
Thus, during electrochemical corrosion, three reactions occur, and in three different places. Anodic occurs in areas of metal loss, cathodic - where oxygen dissolved in water can accept electrons, and solid scale itself is formed in places of mechanical damage on the surface of the product.
Recently, another type of corrosion has been identified - mechanochemical, which occurs as a result of the dynamic interaction of contacting environmental elements under conditions of high contact pressures.
Protection of metal materials from corrosion in neutral aerated environments
Use of sustainable metals and alloys
- with increased thermodynamic stability, for example, copper and alloys based on it (brass and bronze), which are quite stable in sea water ( j Cu = + 0.35 V
), copper is used both in the form of pure metal and in the form of alloys with other metals - with zinc (brass), zinc and aluminum, tin or nickel (special brasses), tin (tin bronze), aluminum (aluminum bronze), nickel (copper-nickel alloy), copper is widely used as a material for fittings of water supply lines and heating systems; - prone to passivation: aluminum (j0 Al = - 1.67 B
) and its alloys (AlMn1, AlMn1Mg1, AlMgSi, AlZn5Mg1, AlZnMgCu, etc.) passivated in oxygen-containing environments with the formation of an oxide film of
Al 2 O 3
or
Al 2 O 3 ∙ H 2 O
(stable in environments with
pH = 3-9
);
the corrosion resistance of pure aluminum decreases if it contains cathode or anodic inclusions; titanium ( j Ti = - 1.63 B
) and its alloys, which have a tendency to transition to a passive state in neutral and oxidizing environments, for example in sea water;
Alloying titanium with components that increase anodic passivability (Mo, Ta, Nb, Zr, Cr) or cathode additives (Pd, Pt, Ru, Re), facilitating the transition to a passive state, makes it possible to obtain alloys with high corrosion resistance not only in neutral environments, but also in acid solutions; nickel ( j Ni = - 0.25 B )
and its alloys with copper, molybdenum and chromium, which are passivated in highly oxidizing environments; these alloys are stable in alkalis of various concentrations, in solutions of many salts, in the atmosphere and in natural waters; Nickel-copper alloys - monels, containing about 30% copper and 3...3% manganese, aluminum, iron, have high strength and corrosion properties; - covered with protective films of secondary, poorly soluble corrosion products ( Zn in H 2 O , Pb
in sulfate solutions).
Removing depolarizer-oxygen from the electrolyte (electrolyte deaeration, deoxygenation
Deaeration is used to remove dissolved oxygen from water.
There are:
- thermal deaeration, in which water is treated with steam in a separate unit; The method is based on the fact that the solubility of oxygen decreases with increasing temperature;
- chemical deaeration, in which dissolved oxygen in water is removed by reaction with sodium sulfite (Na2SO3) or hydrazine (N2H4) according to the following equations:
Introduction of various additives into the electrolyte that slow down the course of corrosion
In one case, this is inhibition of the anodic process due to the introduction of passivating substances into the electrolyte ( K 2 CrO 4
, NaNo 2
, etc.). In addition, substances called corrosion inhibitors (IC) are used.
They slow down the corrosion of metals in certain corrosive environments and impart protective properties when introduced into substances or materials.
There are inhibitors of acid corrosion, alkali solutions, non-aqueous media (gasoline, oil and others), neutral media and atmospheric corrosion.
In particular, anodic inhibitors that affect the anodic reaction are used as corrosion inhibitors for neutral environments.
Some anodic inhibitors, such as chromate ions ( CrO 4 2- )
and nitrite ions
( NO 2 - )
, and in the presence of air, phosphates and molybdates, act to cause the formation of a protective (passive) oxide layer on the surface of the steel.
But if the inhibitor concentration is too low, pores and defects may appear in the oxide layer, where accelerated corrosion may occur. Therefore, such inhibitors are called “dangerous inhibitors.”
Creation of protective coatings on the surface of metal materials:
Metal ( Zn -, Cd -, Ni -, Pb
— coatings on the surface of steel products);
According to the method of protective action, metal coatings are divided into cathodic and anodic.
Cathode coatings made of Pb , Cu , Ni
created on the surface of steel products protect the latter purely mechanically, because
of their electrode potential ( jp )
is greater than that
of steel ( jp > jFe )
. Therefore, the main requirement for them is their non-porous nature.
Anodic coatings made of Zn
, Cd
, due to the fact that
j p < j Fe
, protect the product not only mechanically, but mainly electrochemically, participating in the anodic process instead of the protected steel product.
The main method of creating metal coatings is galvanic. In recent years, spraying methods have become widespread - plasma, gas-phase, thermal diffusion, etc.
Non-metallic inorganic;
- Non-metallic inorganic coatings include oxide and phosphate.
- Oxide coatings are created by the oxidation method - the creation of an oxide film on the surface of the protected metal product, which appears during the process of anodic dissolution of the surface of the protected metal.
For example, the oxidation of aluminum comes down to a reaction of the form:
2 Al
+ 3 H 2 0 = Al 2 O 3 + 3 H 2 .
Oxidation of ferrous metals – bluing. It is carried out by chemical, thermochemical and electrochemical methods. A film of magnetic iron oxide Fe3O4 forms on iron and its alloys. Its durability is low, so it performs mainly decorative functions.
Phosphate coatings are created in the process of phosphating - processing steel and aluminum products in a hot solution of phosphate salts Fe, Zn.
In this case, a film of sparingly soluble phosphates (FeHPO4 or Fe3(PO4)2) is formed on the surface of the steel product. The film is porous and “absorbs” oil and paint well, reliably protecting the steel from corrosion. Phosphating is mainly applied to steel.
There are several process options: the most commonly used are zinc phosphating, iron phosphating or sodium ammonium phosphating; There is also manganese phosphating.
In zinc phosphating, the steel surface is treated in a bath containing H3PO4, acid phosphate, zinc ions and some additives, such as fluorides, nickel ions and organic compounds.
During processing, oxidation and some dissolution of iron from the surface occur, and the pH near it increases slightly. As a result, sparingly soluble iron-zinc or zinc phosphates are deposited on the surface. The mass of the coating, depending on the conditions, ranges from 0.2 to 30 g/cm3.
By itself, the phosphate coating provides little protection against corrosion, but in combination with subsequent impregnation with oil it provides good protection, since the porous coating can absorb a significant amount of oil. The method is used to protect weapons, hammer drills and some machine parts.
The combination of phosphating and anti-corrosion painting is widely used for cold-rolled steel sheet products such as automobile bodies.
Iron or sodium ammonium phosphating is used only for steels. It is carried out in a bath containing sodium dihydrogen phosphate (NaH2PO4) or ammonium dihydrogen phosphate (NH4H2PO4) at pH = 4.0...5.5.
The resulting coating consists of iron phosphate (Fe3(PO4)2 8H2O), magnetite (Fe3O4) and some iron-chromium compounds - the result of a final wash with a solution containing chromic acid - H2CrO4 and Cr3+ ions.
It has a mass of 0.2 to 1.0 g/m2 and, depending on conditions, can be yellow-green, purple, blue or gray.
Iron phosphating is carried out as a preparation for painting to improve the adhesion of the paint layer to the base. The method is applicable for sheet metal structures, for example, for household machines.
Corrosion protection methods
Rust and other corrosive effects can lead to safety issues and compromise the integrity of production equipment and supplies. Even routine maintenance to remove and remove rust increases operating costs. A number of methods have been developed that can be used to minimize corrosion.
Methods for removing corrosion
If rust has already appeared, it can be removed in different ways - mechanically, chemically. You can also use folk remedies.
Rusty castle (Photo: pixabay.com)
Mechanical cleaning
Involves the use of abrasive tools. Damaged parts will be cleaned by friction.
Brush on metal
It is a classic hand brush with many metal fibers used for cleaning. Suitable for partial removal of the effects of corrosion.
Metal coatings
These methods of preventing corrosion involve immersing the steel in a molten metal whose electrical potential is lower than that of iron (the greater the difference, the more effective the coating).
Practical applications include electroplating with zinc or tin, as well as diffusion coatings with nickel, chromium, silicon or aluminum. Compared to other corrosion protection methods, galvanization is known for its lower initial costs, durability and versatility.
Since the consumption of protector metal is quite high, technologies that are characterized by the efficiency of the components used and the strength of the coatings created are advantageous. First on this list is galvanizing. The iron in the steel reacts with the zinc to form a strong alloy coating that serves as protection.
Effective metal protection with zinc coating
To extend the service life of metal products and structures, it is necessary to ensure their protection from moisture and other external influences. To give the metal anti-corrosion properties, a protective zinc coating is used. This treatment is called galvanizing. The technology of coating various metals with zinc was invented more than 200 years ago, and it is still actively used today, due to the high protection efficiency and durability of the anti-corrosion layer.
Various application methods are used - hot, galvanic, gas-dynamic, diffuse, cold galvanizing. Metal coated with a thin layer of zinc (80-200 microns) does not rust for more than 50 years. The zinc coating wears out over time and must be renewed. Depending on the operating conditions of the metal structure, the rate of loss of zinc surface is 1-6 microns per year. For comparison, paint and varnish coatings that protect metal must be renewed at least once every 5 years.
Properties of zinc coatings:
- High degree of corrosion protection.
- Electrochemical (cathodic) protection of metal products and structures.
Non-metallic coatings
One of the easiest ways to prevent corrosion is to use non-metallic protective coatings such as paint, plastic, wax or powder. Powders, including epoxy resin, nylon and urethane, are applied to a metal surface and heated to the melting stage, forming a thin film.
The paint acts as a coating that protects the metal surface from the electrochemical charge that comes from corrosive compounds. Typically they use a combination of different layers of paint that perform different functions. The primer acts as an inhibitor, the intermediate coat increases the overall thickness of the paint, and the topcoat provides resistance to environmental factors.
Basic galvanizing methods
Zinc coating is the best method for protecting iron surfaces from corrosion.
Galvanizing is performed in the following ways:
- Hot galvanizing. Immersion of iron sheets, sections or shaped metal products into molten zinc, the temperature of which is 460-480 degrees. This technology allows you to protect metal from corrosion for a long time, but it is difficult and unsafe to implement. Other disadvantages include: limitation of processing by the size of the baths, the possibility of deformation of thin structures and sheets when heated, damage to the protective layer during welding.
- Cold galvanizing. It is considered the optimal way to protect metal with zinc. It is performed by painting metal surfaces with powdered primer with 96-98% zinc content. The coating is applied with a roller or brush directly at the installation site of the structure (i.e., for anti-corrosion protection, there is no need to transport the product). Cold galvanizing makes it possible to protect iron from rust for 30-50 years; under a layer of zinc primer, the metal corrodes three times slower compared to other processing methods. Other advantages of this technology include cost-effectiveness (compared to hot-dip galvanizing). Disadvantages: difficulty in covering uneven surfaces and internal cavities.
- Gas-thermal method. Application of molten zinc to a metal surface in a gas stream. This technology is suitable for large-sized metal structures that do not fit in a bath of zinc solution. The coating lasts for 25-30 years. The disadvantages of the technology are the unevenness of the resulting coating, which is complemented by the application of paint and varnish.
- Thermal diffusion method. Melting zinc atoms into iron at high temperatures (over 2600 degrees). At this temperature, zinc goes into a gaseous state, after which diffusion of zinc molecules with the metal occurs. Advantages of the method: high class of anti-corrosion protection, preservation of the product configuration, the ability to adjust the thickness of the zinc coating, no need to clean up waste. Disadvantages: heterogeneity of the thickness of the protective film, low productivity and harmfulness of the technological process.
- Galvanic method. Electrolytic galvanizing method, which allows you to apply a thin (5-40 microns) layer of zinc to a degreased metal surface. It consists of placing metal and zinc plates in an electrolytic solution and connecting an electric current. Zinc dissolves in the electrolyte and settles on the iron in the form of a protective layer. It is distinguished by the uniformity and smoothness of the coating layer, including hardware of complex configurations and porous surfaces. Disadvantages: high cost, the need to clean the waste before discharging it into the sewer.
The choice of galvanizing technology depends on the requirements for the technical characteristics of the coating, operating conditions of metal products or structures. If you have questions about how zinc protects metal from corrosion and which galvanizing method is suitable for a particular type of rolled metal, you can get advice from a specialist from our company.
Chemical coatings
Refers to methods of temporary anti-corrosion protection of steel, for example, during plastic deformation at elevated temperatures. The most widely used technologies are phosphating and oxalation.
When phosphating, the surface is covered with a continuous layer of phosphate salts of iron and manganese, and when oxalating, it is covered with water-soluble salts of oxalic acid. Phosphating is used for processing unalloyed steels, oxalation – for alloyed ones. The coating adheres firmly to the surface, helping to reduce friction and reduce tool wear. After stamping is completed, the coating is removed.
Changes in the composition of technical metal and corrosive environment
It consists of special alloying of steel with elements that increase its corrosion resistance. If possible, a lubricant containing anti-corrosion components (reducing agents) is introduced into the mechanical system, which operates in conditions of high temperature and humidity.
An element that has a positive effect on the corrosion resistance of steel is chromium. To realize this effect, steel must contain at least 13% chromium. Every additional 5% chromium provides even better corrosion resistance.
Nickel is the second important element for improving the corrosion resistance of steel, and the addition of nickel also leads to stabilization of austenite. The third important element for increasing corrosion resistance is molybdenum. However, its additives increase the corrosion resistance only of stainless steels with sufficient chromium and nickel content.
Electrochemical protection
The corrosion process, which occurs when two different metals in an electrolyte come into contact, can be stopped using a cathodic protection system. To implement the method, active centers on the metal surface must be converted into passive ones by providing electrons from another source (usually anodes attached to the surface are used). The metals used for anodes are aluminum, magnesium or zinc.
Cathodic protection is very effective in home appliances, but the anodes must be checked frequently, which increases maintenance costs.