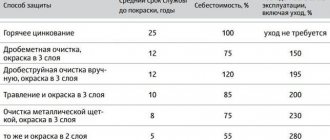
Electrochemical protection – an effective way to protect finished products from electrochemical corrosion. In some cases it is impossible to renew the paint coating or protective wrapping material, then it is advisable to use electrochemical protection. The coating of an underground pipeline or the bottom of a sea vessel is very labor-intensive and expensive to renew, sometimes simply impossible. Electrochemical protection reliably protects the product from corrosion, preventing the destruction of underground pipelines, ship bottoms, various tanks, etc.
Electrochemical protection is used in cases where the potential for free corrosion is in the area of intense dissolution of the base metal or repassivation. Those. when there is intense destruction of metal structures.
The essence of electrochemical protection
A direct current (DC source or protector) is connected to the finished metal product from the outside. Electric current on the surface of the protected product creates cathodic polarization of the electrodes of microgalvanic pairs. The result of this is that the anodic areas on the metal surface become cathodic. And due to the influence of a corrosive environment, it is not the metal of the structure that is destroyed, but the anode.
Depending on which direction (positive or negative) the metal potential shifts, electrochemical protection is divided into anodic and cathodic.
Combination of protectors and paints
Often there is a need to protect a gas pipeline from corrosion not only with a protector, but with paint and varnish material. Paint is considered a passive method of protection against corrosion processes and is truly effective only when combined with the use of a protector.
This combination technique allows:
- Reduce the negative impact of potential defects in the coating of metal structures (peeling, swelling, cracking, heaving, etc.). Such defects occur not only as a result of manufacturing defects, but also due to natural factors.
- Reduce (sometimes by a very significant amount) the consumption of expensive protectors, while increasing their service life.
- Make the distribution of the protective layer over the metal more uniform.
It is also worth noting that paint and varnish compositions are often not easy to apply to certain surfaces of an already operating gas pipeline, tanker or some other metal structure. In such cases, you will have to make do with only a protective protector.
Cathodic corrosion protection
Cathodic electrochemical corrosion protection is used when the metal being protected is not prone to passivation. This is one of the main types of protection of metals from corrosion. The essence of cathodic protection is the application of an external current to the product from the negative pole, which polarizes the cathode sections of the corrosive elements, bringing the potential value closer to the anodic ones. The positive pole of the current source is connected to the anode. In this case, corrosion of the protected structure is almost reduced to zero. The anode gradually deteriorates and must be replaced periodically.
There are several options for cathodic protection: polarization from an external source of electric current; reducing the rate of the cathodic process (for example, deaeration of the electrolyte); contact with a metal whose free corrosion potential in a given environment is more electronegative (so-called sacrificial protection).
Polarization from an external source of electric current is used very often to protect structures located in soil, water (bottoms of ships, etc.). In addition, this type of corrosion protection is used for zinc, tin, aluminum and its alloys, titanium, copper and its alloys, lead, as well as high-chromium, carbon, alloy (both low and high alloy) steels.
The external current source is cathodic protection stations, which consist of a rectifier (converter), a current supply to the protected structure, anode grounding conductors, a reference electrode and an anode cable.
Cathodic protection is used as an independent or additional type of corrosion protection.
The main criterion by which one can judge the effectiveness of cathodic protection is the protective potential. Protective potential is the potential at which the corrosion rate of a metal under certain environmental conditions takes on the lowest (as far as possible) value.
There are disadvantages to using cathodic protection. One of them is the danger of overprotection. Overprotection is observed with a large shift in the potential of the protected object in the negative direction. At the same time it stands out. The result is the destruction of protective coatings, hydrogen embrittlement of the metal, and corrosion cracking.
Causes of corrosion
Since the electrochemical method of protecting a car is aimed exclusively against corrosion, the reasons that cause it to damage the body should be considered. The main ones are water and road reagents used during the cold period. When combined with each other, they form a highly concentrated salt solution. In addition, dirt settled on the body retains moisture in the pores for a long time, and if it contains road reagents, it also attracts water molecules from the air.
The situation is aggravated if the car's paintwork has defects, even small ones. In this case, the spread of corrosion will occur very quickly, and even the remaining protective coatings in the form of primer and galvanization may not stop this process. Therefore, it is important not only to constantly clean the car from dirt, but also to monitor the condition of its paintwork. Temperature fluctuations as well as vibrations also play a role in the spread of corrosion.
You should also note the areas of the car that are most susceptible to corrosion. These include:
- parts located closest to the road surface, that is, sills, fenders and underbody;
- welds remaining after repairs, especially if they were carried out incorrectly. This is explained by high-temperature “weakening” of the metal;
- in addition, rust often affects various hidden, poorly ventilated cavities where moisture accumulates and does not dry out for a long time.
Tread protection (use of protector)
A type of cathodic protection is sacrificial. When using sacrificial protection, a metal with a more electronegative potential is connected to the protected object. In this case, it is not the structure that is destroyed, but the tread. Over time, the protector corrodes and must be replaced with a new one.
Tread protection is effective in cases where there is a small transition resistance between the protector and the environment.
Each protector has its own radius of protective action, which is determined by the maximum possible distance to which the protector can be removed without losing the protective effect. Protective protection is most often used when it is impossible or difficult and expensive to supply current to the structure.
Protectors are used to protect structures in neutral environments (sea or river water, air, soil, etc.).
The following metals are used to make protectors: magnesium, zinc, iron, aluminum. Pure metals do not fully perform their protective functions, so they are additionally alloyed during the manufacture of protectors.
Iron protectors are made from carbon steel or pure iron.
Zinc protectors
Zinc protectors contain about 0.001 - 0.005% lead, copper and iron, 0.1 - 0.5% aluminum and 0.025 - 0.15% cadmium. Zinc projectors are used to protect products from sea corrosion (in salt water). If a zinc protector is used in slightly salted, fresh water or soils, it quickly becomes covered with a thick layer of oxides and hydroxides.
Magnesium protector
Alloys for the manufacture of magnesium protectors are alloyed with 2–5% zinc and 5–7% aluminum. The amount of copper, lead, iron, silicon, nickel in the alloy should not exceed tenths and hundredths of a percent.
Magnesium protector is used in slightly salted, fresh waters and soils. The protector is used in environments where zinc and aluminum protectors are ineffective. An important aspect is that magnesium protectors must be used in an environment with a pH of 9.5 - 10.5. This is explained by the high rate of dissolution of magnesium and the formation of sparingly soluble compounds on its surface.
Magnesium protector is dangerous because... is the cause of hydrogen embrittlement and corrosion cracking of structures.
Aluminum protectors
Aluminum protectors contain additives that prevent the formation of aluminum oxides. Up to 8% zinc, up to 5% magnesium and tenths to hundredths of silicon, cadmium, indium, and thallium are added to such protectors. Aluminum protectors are used in the coastal shelf and flowing sea water.
Cathode polarization technology
In this case, the so-called superimposed current is used. An external conductor (often) or a current source (rarely) is used to apply it to a metal object. Upon contact with an electrically active particle, the following happens: the particle, under the influence of electrical attraction forces, moves to a protective element with a negative charge, where “recycling” of these particles occurs.
The consequences of such “disposal” are obvious - the protective element itself becomes corroded over time and becomes unusable. Therefore, this technology is often called the sacrificial electrode method (instead of our part, the “sacrificial electrode” rusts).
In addition to current and voltage, when working with cathodic polarization, one more important parameter must be taken into account - the ohmic voltage. In a technical sense, this parameter reflects the fact that as an electrical charge flows over time, the current voltage in the circuit drops. The drop itself occurs due to the fact that the cathode current flows along a circuit with a lower charge. If the circuit is assembled correctly, this indicator is quite small - thanks to this, the same current of the same power will always be maintained in the circuit.
grounding
Power supply UNP2-7-65
The housings of the switchboard, UNP installation, compressor, and air heater are united by a common grounding wire, which is connected to a grounding bolt mounted on the car frame on the left side. This bolt must be connected to the s.
Air heater for UNP2-7-65
2. Check the ground connection to the control panel. 6.3. Open the control panel. Make sure there is no moisture or dirt inside the control panel and check the position of the handles of the RCD switches and the “Heating” automatic device: the RCD must be turned on (knob .
Installation of intra-shop pipelines
What are the minimum distances allowed between the axes of the pipes being laid? 4. Tell us about the rules for grounding pipelines to remove static electricity. .
History of discovery
Cathodic protection was first described by Sir Humphry Davy in a series of papers presented to the Royal Society of London for the Advancement of Natural Knowledge in 1824. After extensive testing, cathodic protection was first used in 1824 on the ship HMS Samarang. Iron anodic protectors were installed on the copper plating of the ship's hull below the waterline, which significantly reduced the rate of copper corrosion. Copper, when corroded, releases copper ions, which have an anti-fouling effect. Due to excessive hull fouling and reduced ship efficiency, the Royal Navy decided to eliminate sacrificial protection to take advantage of the anti-fouling effect due to copper corrosion.
Protection of hidden car cavities
Protection of hidden cavities
In order to protect hidden cavities, you need to know several rules. High-quality processing of hidden cavities is one of the key points in car protection. A real specialist who works at a service station can select reliable protection for these places.
In this case, mastic is not the best method of protection. At the moment, there are a lot of chemicals that will protect your car and prevent rust from appearing on the body and more. Modern companies are developing materials that penetrate the surface of the body and create a protective film; cracks should be treated with anti-corrosion coating agents.
Electronic protection method
Electronic protection method
Electronic protection of a car against corrosion is capable of significantly slowing down the process of corrosion formation in 99.7% of cases. This is evidenced by numerous tests that were conducted by scientists. This protection helps protect your car from corrosion for up to ten years. Many companies provide these devices for sale and provide a guarantee on them. They do not create interference while listening to radio stations; they meet all modern requirements and quality standards.
This device is quite easy to install; many people install electronic protection on their own, without the help of specialists. You should make sure that all the wires of the device are in place and do not interfere with the car owner.
Plastic fasteners or insulating tape should be used for wires. You can check the operation of the device by the glowing lights; they should light up when you turn on the engine; if this does not happen, then you should check the operation of the battery. If even after this the light does not light up, then you should contact the dealer from whom you purchased the device.
Advantages of the electronic method
Electronic protection of your car against corrosion will help you significantly save money and time. After all, the most vulnerable to corrosion are the rear fenders, the body, the bottom, and the inner surface of the transmission. It is in these places that corrosion develops frequently and rapidly, mainly due to poor ventilation and accumulation of moist air.
When installing fender liners, it is worth ensuring freedom of air access to ensure ventilation. The main disadvantage of mastic is that it has to be treated with the car every year, unlike electronic protection. Also, pillars, internal beams, side members, and the ceiling are no less vulnerable. In order to process them, you need to drill special holes, and this is an additional source of penetration of harmful substances into the cavities of the car.
Various corrosion can occur during mechanical damage to the body. Such damage can destroy the structure of the metal, and this is extremely dangerous for the integrity of your car. Many parts will simply have to be replaced, since they cannot be repaired.
Electronic protection of a car against corrosion is practically the only method of protection against rust with internal stresses; it completely eliminates the impact of weather conditions on the metal. This protection is compatible with any mastic and additional anti-corrosion agent. Your body will be under reliable protection.
All of the above proposed protection methods will help protect your vehicle from corrosion, they will be able to eliminate its occurrence. You can treat your car yourself with mastic or primer, as well as anti-corrosion agents.
We also have specialists at your service who will help you install electronic or electrochemical protection at a high professional level. Your car will be in reliable and strong hands of a true master of his craft.
fCcyleEGWCA
Rules for gutting and cutting pike
The pike is gutted and cut after cleaning the scales.
How to properly cut pike into cutlets:
The belly of the fish is cut open and the entrails are pulled out.
It is important not to damage the bile, otherwise the product may be spoiled. The esophagus is removed along with the gills, leaving the head
The white film running along the belly (also known as the swim bladder) is removed. The head is cut off with a knife.
After that, they move on to cutting up the carcass itself. This procedure will vary depending on what you plan to cook: minced, filleted, whole pieces or stuffed fish.
For minced meat
If you plan to prepare minced meat for cutlets, only large bones (spine) are removed. The vertebral column of the pike is strong and hard. If the pike is small, then the spine does not need to be removed.
Cutting medium and large pike for minced meat involves the following steps:
- The fish is placed on a cutting board.
- The knife blade is placed parallel to the spine (in the place where the head was) and the fillet is cut to the tail. The knife should be held as close to the ridge as possible.
- In the same way, cut the fillet from the other side of the fish.
- The middle part with the spine is removed; it will be useful for preparing fish soup.
- The pike for cutlets is deboned, but not thoroughly, because... During the process of grinding meat into minced meat, the bones crumble and are not felt in the finished cutlets. The fillet is scrolled in a meat grinder through a fine grill. To better grind the seeds, this can be done twice.
For frying fillets
There are many bones in the pike's body, among them there are massive (spine), medium and very thin. There are a lot of seeds and it is difficult to remove them.
The boneless product is prepared in the following order:
- The fillet is cut along the entire length of the carcass.
- The pike is turned over and the fillet is cut off on the other side.
- The remaining ridge and tail section are cut into pieces and set aside.
- The ribs are cut from the fillet. They are very thin; if the knife is sharp, there will be no significant loss of product.
- Fins are cut out.
- The flesh with the bones is felt above the rib line and two parallel cuts are made along the fillet.
- The strip with bones is separated with a knife and cut out of the fillet.
- The remaining bones are removed with tweezers. A layer of boneless fish pulp is obtained.
- The skin is removed from the fish.
- The fillet is cut into portions and the dish is prepared according to the chosen recipe.
For frying in pieces
Preparation for frying in pieces does not take much time.
The carcass is processed as follows.
- The fins are cut off from the pike.
- The tail is cut off.
- Cut the fish into pieces 1.5-2 cm thick. If you plan to bake the pike in the oven, the length of the pieces can reach 10-15 cm.
- If the pike is large, the pieces are cut randomly to make it convenient to place them in the pan.
For stuffing
Stuffed pike (with and without head) is prepared in the oven. Medium-sized fish are suitable for this dish.
Cutting steps:
- An incision is made around the pike head.
- The skin along with the fins is removed from the carcass using a knife and scissors. To ensure that the skin is easily removed, the carcass is beaten with a rolling pin.
- The pike meat is cut off (and picked by hand) from the remaining part of the carcass. All that remains is the skeleton of the fish.
- Prepare minced meat for filling using fillet, vegetables, herbs, meat, eggs, and other ingredients.
- The removed skin is stuffed with minced meat, and the abdomen is sewn up. The dish is prepared in the oven.