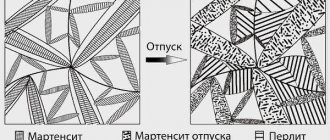
Tempering is a heat treatment process consisting of heating hardened steel to temperatures below the Ac1 point in order to obtain an equilibrium structure and a given set of mechanical properties.
After hardening, the steel has a structure based on martensite with a tetragonal distorted crystal lattice and retained austenite, the amount of which depends on the chemical composition of the steel. When hardened steel is heated, phase transformations occur in its structure, which can be shown in the form of a diagram.
Scheme of phase transformations during steel tempering
Low steel tempering
Low tempering of steel is done at temperatures up to 250°C. During this process, some of the excess carbon is released from martensite to form tiny carbide particles (ε-carbides). ε-carbides precipitate in the form of plates or rods and they are coherently bonded to the martensite lattice. The decomposition of retained austenite during low tempering occurs according to the mechanism of bainite transformation: a heterogeneous mixture of low-carbon martensite crystals and dispersed carbides is formed. The product of low tempering is tempered martensite, which differs from quenched martensite by its lower carbon concentration and the presence of carbides (ε-carbides) in it, which are coherently bonded to the martensite lattice.
At a temperature of about 250°C, the transformation of carbide into cementite begins; in this case, the coherence of the lattices of the α-solid solution of martensite and carbides is disrupted.
Iron-carbon tool materials (cutting and measuring tools), as well as steels that have been carburized or nitrocarburized, are subjected to low tempering. Low tempering is often done for steels after heat treatment with high frequency currents.
Laxative effect of sorbitol (sorbitol)
Dispensing steel
Sorbitol has a pronounced laxative effect, increasing in proportion to the amount taken into the body. The recommended daily dose is 30-40 grams per day (determined individually). Doses within 30-50 (determined individually) grams cause flatulence. Doses over 45-50 grams (determined individually) lead to a strong laxative effect, accompanied by flatulence.
Sorbitol is used as a medicine to combat constipation in laxative preparations in the form of chocolates and candies.
Sorbitol can be used as a laxative when taken orally or as an enema. Sorbitol works as a laxative by drawing water into the colon, stimulating bowel movements. [ source unspecified 73 days
]
Average holiday
Average tempering is carried out at temperatures of 350–400 °C. In this case, all excess carbon is released from martensite with the formation of cementite particles. The tetragonality (degree of tetragonality) of the iron lattice decreases, it becomes cubic. As a result, ferrite remains instead of martensite. Such a ferrite-cementite mixture is called tempering troostite, and the process leading to such changes is called medium-temperature tempering. With medium tempering, the dislocation density decreases and the internal stresses in the steel decrease.
Medium tempering is used for heat treatment of elastic parts: leaf springs, springs, etc.
Expanded perlite
Steel tempering
Perlite is also called acidic volcanic glass with a fine structure, along which it splits into small balls, sometimes having a pearly luster. The composition of such expanded perlite,%: SiO2 65-75; Al2O3 10-15; Fe2O3 1.5-2.5; CaO 1.5-2.5; MgO 1.5-2.0. Expanded perlite
contains up to 3-6% constitutional (bound) water. When heated quickly, the water contained in this perlite evaporates, swelling the rock with an increase in volume up to 10-20 times. Swelling temperature 850-1200°C. Expanded perlite has a volumetric mass of 70-600 kg/m3, which allows it to be used as a lightweight filler in thermal insulation products.
Expanded perlite is used primarily in construction: in the production of effective plaster, bricks and blocks from artificial perlite stone (the advantages of which are low weight and ease of processing), as a soundproofing filler, insulation, etc. In addition, expanded perlite is used in agriculture and more.
Lit.:
Gulyaev A.P. Metallurgy. - M.: Metallurgy, 1977. - UDC669.0 (075.8)
Ivanov V.N. Dictionary-reference book for foundry production. – M.: Mechanical Engineering, 1990. – 384 p.: ill. ISBN 5-217-00241-1
Zimmerman R., Gunter K. Metallurgy and materials science. Reference ed. Per. with him. M.: Metallurgy, 1982. 480 p.
See also Iron-carbon alloys and Isothermal transformation of austenite.
High holiday
During high tempering (450-550°C and above), structural changes occur in carbon steels that are not associated with phase transformations: the shape, size of carbides and ferrite structure change. With increasing temperature, coagulation occurs - the enlargement of cementite particles. The shape of the crystals gradually becomes spherical - this process is called spheroidization.
Coagulation and spheroidization of carbides begin to occur more intensely at a temperature of 400°C. The ferrite grains become large and their shape approaches equiaxial. The ferrite-carbide mixture, which is formed after tempering at a temperature of 400–600 ° C, is called tempering sorbitol. At a temperature close to point A1, a fairly coarse ferrite-cementite mixture is formed - pearlite.
High tempering at temperatures of 450-550°C is used for most structural steels. It is widely used in the heat treatment of various bushings, supports, fasteners operating in tension-compression and other products that experience static loads.
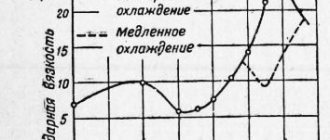
Temper brittleness phenomenon
When tempering some steels, processes may occur that reduce the impact strength of the steel without changing other mechanical properties. This phenomenon is called temper brittleness and is observed in the tempering temperature ranges at 250–400ºС and 500–550ºС. The first type of brittleness is called type 1 temper brittleness and is irreversible, so tempering steels at these temperatures should be avoided. This type is inherent in almost all steels alloyed with chromium, magnesium, nickel and their combination, and is due to the heterogeneous precipitation of carbides from martensite. The second type of temper brittleness, temper brittleness of the ΙΙth kind, is reversible. Temper brittleness of the ΙΙ-th kind manifests itself when alloy steel is slowly cooled at a temperature of 500–550°C. This brittleness can be eliminated by repeated tempering at a high cooling rate (in water or oil). In this case, the cause of this brittleness is eliminated—the precipitation of carbides, nitrides, and phosphides along the boundaries of former austenite grains. It is possible to eliminate the temper brittleness of alloy steels by introducing small additions of molybdenum (0.2–0.3%) or tungsten (0.5–0.7%) into them.
Graphically, these types of fragility look as shown in the figure.
Manifestation of temper brittleness in steels during tempering
Almost all steels obey the law: an increase in tempering temperature leads to a decrease in strength characteristics and an increase in plasticity, as shown in the figure below.
Effect of tempering temperature on the mechanical properties of steel
This pattern does not apply to high-speed tool steels alloyed with carbide-forming elements.
Structure - troostite
Application of alloy steels. classification and marking of alloys
The troostite structure is formed during slower cooling and is a highly dispersed mixture of ferrite and cementite. Troostite has lower hardness and strength than martensite.
The troostite structure is formed as a result of the transformation of austenite and is a highly dispersed mixture of ferrite and cementite. Acicular troostite is called bainite. Troostite is produced by slower cooling and has lower hardness and strength than martensite.
The troostite structure is formed during slower cooling and is a highly dispersed mixture of ferrite and cementite. Troostite has lower hardness and strength than martensite.
The troostite structure is formed as a result of the transformation of austeite and is a highly dispersed mixture of ferrite and cementite. Acicular troostt is called bainite. Troostite is obtained by slower cooling and has lower hardness and strength than martensite.
Steel with a troostite structure has increased hardness 40 - 45 HRC, strength and moderate toughness and ductility.
Carbide particles in the structure of troostite or tempered sorbitol, in contrast to troostite and sorbitol obtained as a result of the decomposition of supercooled austenite, have a granular rather than lamellar structure. The formation of granular structures improves many properties of steel, especially ductility and toughness, and most importantly, resistance to fracture. With the same hardness and tensile strength, steel with a grain structure has higher values of yield strength, relative contraction and impact strength, as well as fracture toughness parameters.
Carbide particles in the structure of troostite or tempered sorbitol, in contrast to troostite and sorbitol obtained as a result of the decomposition of supercooled austenite, have a granular rather than lamellar structure. The formation of grain structures improves many properties of steel. With the same hardness, tensile strength and ductility, steel with a granular structure has higher values of yield strength, relative contraction and impact strength.
Carbide particles in the structure of troostite or tempered sorbitol, in contrast to troostite and sorbitol obtained as a result of the decomposition of supercooled austenite, have a granular rather than lamellar structure. The formation of granular structures improves many properties of steel, especially ductility and toughness, and most importantly, resistance to fracture. With the same hardness and tensile strength, steel with a grain structure has higher values of yield strength, relative contraction and impact strength, as well as fracture toughness parameters.
When martensite decomposes, the structures of troostite, sorbitol and pearlite are obtained. They differ from the same structures obtained during the decomposition of austenite in particle size and mechanical properties. The shape of cementite inclusions formed during the decomposition of martensite is round, while the decomposition of austenite produces cementite plates. Different shapes of cementite inclusions determine different properties. With the same strength, steel after tempering becomes more ductile.
Above 350 C, tempered troostite structures are formed, and with a further increase in heating temperatures, tempered sorbitol structures are formed.
Tempered troostite and sorbitol differ from the structure of troostite and hardened sorbitol. While the latter have a lamellar structure, troostite and tempered sorbitol have a granular structure.
| Structure of tempered steel (0 6% C, X 500. a - tempered martensite. b - troostite. c - sorbitol. |
With the structure of troostite or sorbitol obtained as a result of heat treatment, no areas with individual grains of structurally isolated ferrite are observed.
Of great interest is alloyed gray cast iron, which has a troostite structure and has high mechanical properties due to a radical change in the structure of the metal base.
The lower limit of the quenching temperature of chromium steel ensures the absence of troostite and coarse carbide accumulations in the structure, and the upper limit limits the onset of the appearance of acicular martensite.
Tempering of high-speed tool steels
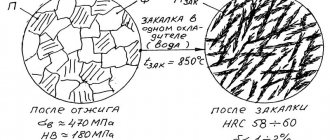
The main alloying elements of high-speed steels (P18, P6M5, etc.) are tungsten, molybdenum, cobalt and vanadium - elements that provide heat resistance and wear resistance during operation. High-speed steels belong to the carbide (ledeburite) class. For hardening, these steels are heated to a temperature above 1200°C (P18 to a temperature of 1270°C, P6M5 to a temperature of 1220°C). High quenching temperatures are necessary for more complete dissolution of secondary carbides and obtaining austenite highly alloyed with chromium, molybdenum, tungsten, and vanadium. This ensures that heat-resistant martensite is obtained after quenching. Even at very high heat, only part of the carbides dissolves. These steels are characterized by the preservation of fine grains at high heating temperatures.
Iron and “quick-cut” alloying elements have very different thermal conductivity properties, therefore, when heating, temperature stops should be made to avoid cracks. Typically at 800 and 1050°C. When heating a large instrument, the first exposure is made at 600°C. The holding time is 5-20 minutes. Holding at the quenching temperature should ensure the dissolution of carbides within the limit of their possible solubility. Cooling of the instrument is most often done in oil. To reduce deformation, stepwise hardening is used in molten salts at a temperature of 400-500°C. The structure of “quick cuts” after quenching consists of highly alloyed martensite containing 0.3-0.4% C, undissolved excess carbides and retained austenite. The higher the quenching temperature, the lower the position of points Mn, Mk and the more retained austenite. In R18 steel there is approximately 25-30% retained austenite, in R6M5 steel - 28-34%. To reduce austenite, cold treatment can be done, but as a rule this is not required.
After quenching, tempering follows at 550 - 570°C, causing the transformation of retained austenite into martensite and dispersion hardening due to the partial decomposition of martensite and the release of dispersed carbides of alloying elements.
This is accompanied by an increase in hardness (secondary hardness). During the holding process during tempering, carbides are released from the retained austenite, which reduces its alloying, and therefore, upon subsequent cooling, it undergoes a martensitic transformation (Mn ~ 150°C). During a single tempering process, only part of the retained austenite is transformed into martensite. In order for all the austenite to transform into martensite, two and three times tempering is used. The holding time is usually 60 minutes. When assigning a regime, it is necessary to take into account the chemical properties of the elements and the frequency of carbide release depending on temperature. For example, the maximum hardness of R6M5 steel is obtained through 3-stage tempering. The first tempering is at a temperature of 350°C, the next two at a temperature of 560-570°C. At a temperature of 350°C, cementite particles are released, evenly distributed in the steel. This contributes to the uniform release and distribution of special M6C carbides at a temperature of 560-570°C. Thinner for enamel - https://www.dcpt.ru
Methods for obtaining austenite
Austenitic steels are formed in the process of the appearance and growth of grains in the initial microstructure of a metal product.
Austenite is formed at the interface between the ferrite and carbide phases. Carbide particles gradually dissolve in the austenite solid solution. Austenite can also be obtained from the eutectoid modification of iron, consisting of ferrite and cementite. To do this, the original metal workpiece must be heated to a temperature of 900 °C
It is important that the alloy contains a minimum carbon concentration of 0.66%. During this process, ferrite transforms into austenite, and cementite is completely dissolved
The result is austenitic stainless steel.
When producing metal blanks from austenitic steels stabilized with titanium, it is necessary to remelt the metal in a vacuum induction furnace. The resulting melt is kept for a long period to denitrogenize it. The amount of time required for this process depends on the weight of the original product. After holding, a mixture of titanium and nitride-forming chemical elements is introduced into the molten austenite.
When adding chromium and nickel to the iron modification, the material must be kept for a longer time. Very often a mixture of molybdenum or phosphorus is added to the resulting solution. These chemicals increase the toughness and fatigue strength of the iron alloy. To reduce the wear of the resulting austenite, additional alloying materials and energy-intensive carbides are used.
Hardenability
The mechanical properties of structural elements depend on the homogeneity of the metal structure, which directly depends on through hardenability and the minimum diameter. This parameter characterizes the formation of more than half of martensite. So the table shows some indicators at which the critical diameter is maintained.
| steel grade | Carrying out hardening at temperature, °C | Critical diameter, mm | |
| Intensive cooling environment | |||
| water | oil | ||
| 45 | 840…850 | until 9 | up to 25 |
| 45G2 | 840…850 | before 18 | up to 34 |
| 40ХН2МА | 840…850 | up to 110 | up to 142 |
| 38Х2МФА | 930 | up to 72 | up to 86 |
As practice shows, alloying elements have a great influence on hardenability. This is especially noticeable in the presence of nickel. Its presence makes it possible to harden parts of large diameter. Thus, a critical part with a diameter of over 100 mm can be turned from steel 40ХН2МА and subjected to heat treatment while maintaining the given properties throughout the entire volume.
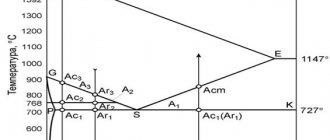
Critical transformation points
Figure 2 shows the cooling and heating curves of pure iron. As can be seen from these curves, during the process of restructuring one lattice into another, as well as during the melting and solidification of iron, temperature stops occur, which are the result of the release of additional heat during cooling and the absorption of additional heat during heating.
Rice. 2. Cooling and heating curves of pure iron.
Temperature stops at which lattice rearrangements occur are called critical temperatures or critical points and are designated Ar during cooling and Ac during heating. At points Ar2 and Ac2, the atomic lattice is not rearranged, but the magnetic properties of iron change. At temperatures above 768°, iron loses its ability to be attracted by a magnet. At a very low rate of heating and cooling, the critical points A c3 and Ar3 do not coincide with each other by 12°. As the cooling rate increases, the discrepancy between the critical points increases, since the temperature decreases significantly and the iron becomes supercooled. This phenomenon is called hysteresis.
When heating and cooling steel, a restructuring of the atomic lattice also occurs, but the temperatures of the critical points are not constant. They depend on the carbon content and alloying impurities in the steel, as well as on the rate of heating and cooling.
In Fig. Figure 3 shows a diagram of the state of carbon steel during slow cooling and heating.
Fig.3. State diagram of carbon steels.