There are two types of temper brittleness:
Temper brittleness of the first type manifests itself during tempering in the temperature range of 250-350 °C for all steels, regardless of their composition and cooling rate after tempering. Therefore, such fragility is called irreversible. It is impossible to eliminate this phenomenon. Therefore, the temperature range of 250-350 °C should be avoided during tempering.
Tempering brittleness of the second type (reversible) is detected after tempering at temperatures above 500 °C. A characteristic feature of this brittleness is that it manifests itself as a result of slow cooling after tempering. Reversible temper brittleness occurs only in some alloy steels, which contain alloying elements such as chromium, manganese, and nickel. This phenomenon occurs in the range of 500-600 °C and only as a result of slow cooling. Therefore, to suppress the development of reversible brittleness, cooling after exposure from the indicated temperatures should be carried out as quickly as possible. A good effect in reducing the tendency to reversible temper embrittlement is achieved by introducing 0.3% molybdenum and 1% tungsten into the steel composition.
It should be borne in mind that, having eliminated temper brittleness by increasing the cooling rate, it can be obtained again if in the future the part made of this steel is operated at temperatures of 500-600 ° C (which is why this brittleness is called reversible brittleness).
Source
Tempering brittleness of metal
/ Articles / Temper brittleness of metal
Most of the known grades of steel have temper brittleness - a special state of the alloy, characterized by a low value of impact strength. Under normal conditions, this property is not capable of influencing other mechanical properties of the material.
The diagram shows a visual representation of the dependence of the tempering temperature on the values of the impact strength of hardened steel, which is characterized by an increased tendency to be in a state of temper brittleness. Most of these materials have two ranges of temper brittleness. During tempering, irreversible brittleness is observed in the range from 250oC to 400oC, and reversible brittleness is observed in the range from 450oC to 650oC.
Correlation of tempering temperature and toughness
The diagram below shows the dependence of the influence of tempering temperature values on the impact strength of a material that has a certain tendency to temper brittleness.
1- The cooling process is carried out at high speed,
2- The cooling process is carried out gradually, at low speed.
The impact strength of various types of steel after tempering in the temperature range from 250oC to 400oC is slightly lower than during tempering at temperatures lower than 250oC.
If, when heating brittle steel, tempered in the range from 250oC to 400oC, to a temperature exceeding 400oC, it is transferred to a viscous state, then the process of secondary tempering in the range 250oC - 400oC will not affect the value of impact strength.
Steel in a state of temper brittleness has a characteristic intergranular fracture localized at the former grain boundaries. Such brittleness is characteristic of all steels, but to varying degrees. It is for this reason that the average tempering of steels is not usually used in practice, but it is this indicator that can provide a large value of the yield strength.
Causes of the phenomenon
One of the main reasons for such a phenomenon as irreversible temper brittleness can be called carbide formation. This term refers to the process that occurs during the decomposition of martensite: the formation of a carbide film at grain boundaries.
These films disappear on their own when heated to a high temperature, and secondary heating from 250oC to 400oC does not lead to their reappearance.
Silicon, present in some steels, helps inhibit the decomposition of martensite.
The impact strength of most types of hardened steels after high tempering in the temperature range from 450oC to 650oC can vary depending on how quickly the cooling process occurs.
As the steel cools gradually from the tempering temperature, the impact strength of most types of hardened steels becomes lower than the value observed after rapid cooling is completed.
The appearance of temper brittleness, observed due to slow cooling during high tempering, is eliminated by repeating the high tempering, however, resorting to high-speed cooling. The impact strength of the material can be reduced again during the next high tempering, and the cooling rate should be slightly lower than at the previous stage.
The elements that make up steel play a significant role in the degree of susceptibility of the material to temper embrittlement. The latter is favored by certain elements, including phosphorus and manganese; the effect of chromium is somewhat weaker.
Chromium-containing steel, which does not contain other additives, is less susceptible to temper brittleness. The addition of manganese, nickel or silicon to the material dramatically increases its susceptibility to temper embrittlement.
In particular, nickel is not capable of causing temper brittleness on its own, however, acting in tandem with chromium or manganese, they contribute to the occurrence of this phenomenon.
Additions of molybdenum and tungsten help reduce the tendency of the material to exhibit temper brittleness. It is molybdenum that has the greatest efficiency, even in small quantities (about 0.2% by weight).
The "dissolution-isolation" theory
Since the creation of structural steels involves significant improvements, reversible temper embrittlement is a rather large difficulty that arises in the way of the manufacturer. There are a number of different theories about the causes of the phenomenon of reversible fragility.
For quite a long time, a huge number of scientists followed the “dissolution-isolation” assumption.
According to this theory, impact strength is reduced due to the appearance of any foreign phases along the grain boundaries, which include phosphides, carbides and other chemical compounds.
After heating the material to a temperature corresponding to high tempering, these phases begin a slow transition into solution, and gradual cooling promotes their release from it, as a result of which the steel loses its strength characteristics.
Rapid cooling of the material from the tempering temperature prevents the formation of new phases that contribute to a decrease in brittle strength. In addition, the “dissolution-release” theory can also explain the reversible nature of temper brittleness.
The interaction of steel with certain substances leads to the etching of grain boundaries in the structure of the material, which are in a state of reversible temper brittleness. The low resistance to some chemicals of these very zones is confirmation of the fact that gradual cooling from high tempering temperatures leads to the occurrence of various structural changes.
In particular, a reduction in impact strength is recorded, but the value of other mechanical characteristics that are measured at room temperature remains unchanged.
Such observations can be explained by the fact that impact strength is a characteristic that is highly dependent on the structure of the material, which is very sensitive to the state in which the grain boundaries are located.
According to L.M. Utevsky, the reversible temper brittleness of alloys is not due to the formation of new types of phases, but to a change in the chemical composition of the solution present in zones near the grain boundaries. For example, filling the above-mentioned zones with phosphorus stimulates a decrease in the work of forming splits between grains, which results in the development of temper brittleness.
- Structural steel
- Tool steel
- Magnetic steel
Tempering of steels
Tempering is a heat treatment process consisting of heating hardened steel to temperatures below the Ac1 point in order to obtain an equilibrium structure and a given set of mechanical properties.
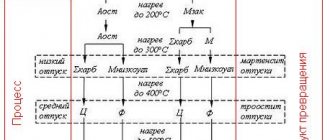
After hardening, the steel has a structure based on martensite with a tetragonal distorted crystal lattice and retained austenite, the amount of which depends on the chemical composition of the steel. When hardened steel is heated, phase transformations occur in its structure, which can be shown in the form of a diagram.
Scheme of phase transformations during steel tempering
Low steel tempering
Low tempering of steel is done at temperatures up to 250°C. During this process, some of the excess carbon is released from martensite to form tiny carbide particles (ε-carbides). ε-carbides precipitate in the form of plates or rods and they are coherently bonded to the martensite lattice.
The decomposition of retained austenite during low tempering occurs according to the mechanism of bainite transformation: a heterogeneous mixture of low-carbon martensite crystals and dispersed carbides is formed.
The product of low tempering is tempered martensite, which differs from quenched martensite by its lower carbon concentration and the presence of carbides (ε-carbides) in it, which are coherently bonded to the martensite lattice.
At a temperature of about 250°C, the transformation of carbide into cementite begins; in this case, the coherence of the lattices of the α-solid solution of martensite and carbides is disrupted.
Iron-carbon tool materials (cutting and measuring tools), as well as steels that have been carburized or nitrocarburized, are subjected to low tempering. Low tempering is often done for steels after heat treatment with high frequency currents.
Average holiday
Average tempering is carried out at temperatures of 350–400 °C. In this case, all excess carbon is released from martensite with the formation of cementite particles. The tetragonality (degree of tetragonality) of the iron lattice decreases, it becomes cubic.
As a result, ferrite remains instead of martensite. Such a ferrite-cementite mixture is called tempering troostite, and the process leading to such changes is called medium-temperature tempering.
With medium tempering, the dislocation density decreases and the internal stresses in the steel decrease.
Medium tempering is used for heat treatment of elastic parts: leaf springs, springs, etc.
High holiday
During high tempering (450-550°C and above), structural changes occur in carbon steels that are not associated with phase transformations: the shape, size of carbides and ferrite structure change. With increasing temperature, coagulation occurs - the enlargement of cementite particles. The shape of the crystals gradually becomes spherical - this process is called spheroidization.
Coagulation and spheroidization of carbides begin to occur more intensely at a temperature of 400°C. The ferrite grains become large and their shape approaches equiaxial. The ferrite-carbide mixture, which is formed after tempering at a temperature of 400–600 ° C, is called tempering sorbitol. At a temperature close to point A1, a fairly coarse ferrite-cementite mixture is formed - pearlite.
High tempering at temperatures of 450-550°C is used for most structural steels. It is widely used in the heat treatment of various bushings, supports, fasteners operating in tension-compression and other products that experience static loads.
Temper brittleness phenomenon
When tempering some steels, processes may occur that reduce the impact strength of the steel without changing other mechanical properties. This phenomenon is called temper brittleness and is observed in the tempering temperature ranges at 250–400ºС and 500–550ºС.
The first type of brittleness is called type 1 temper brittleness and is irreversible, so tempering steels at these temperatures should be avoided. This type is inherent in almost all steels alloyed with chromium, magnesium, nickel and their combination, and is due to the heterogeneous precipitation of carbides from martensite.
The second type of temper brittleness, temper brittleness of the ΙΙth kind, is reversible. Temper brittleness of the ΙΙ-th kind manifests itself when alloy steel is slowly cooled at a temperature of 500–550°C. This brittleness can be eliminated by repeated tempering at a high cooling rate (in water or oil).
In this case, the cause of this brittleness is eliminated—the precipitation of carbides, nitrides, and phosphides along the boundaries of former austenite grains. It is possible to eliminate the temper brittleness of alloy steels by introducing small additions of molybdenum (0.2–0.3%) or tungsten (0.5–0.7%) into them.
Graphically, these types of fragility look as shown in the figure.
Manifestation of temper brittleness in steels during tempering
Almost all steels obey the law: an increase in tempering temperature leads to a decrease in strength characteristics and an increase in plasticity, as shown in the figure below.
Effect of tempering temperature on the mechanical properties of steel
This pattern does not apply to high-speed tool steels alloyed with carbide-forming elements.
Tempering of high-speed tool steels
The main alloying elements of high-speed steels (P18, P6M5, etc.) are tungsten, molybdenum, cobalt and vanadium - elements that provide heat resistance and wear resistance during operation. High-speed steels belong to the carbide (ledeburite) class. For hardening, these steels are heated to a temperature above 1200°C (P18 to a temperature of 1270°C, P6M5 to a temperature of 1220°C).
High quenching temperatures are necessary for more complete dissolution of secondary carbides and obtaining austenite highly alloyed with chromium, molybdenum, tungsten, and vanadium. This ensures that heat-resistant martensite is obtained after quenching. Even at very high heat, only part of the carbides dissolves.
These steels are characterized by the preservation of fine grains at high heating temperatures.
Iron and “quick-cut” alloying elements have very different thermal conductivity properties, therefore, when heating, temperature stops should be made to avoid cracks. Typically at 800 and 1050°C. When heating a large instrument, the first exposure is made at 600°C. The holding time is 5-20 minutes.
Holding at the quenching temperature should ensure the dissolution of carbides within the limit of their possible solubility. Cooling of the instrument is most often done in oil. To reduce deformation, stepwise hardening is used in molten salts at a temperature of 400-500°C.
The structure of “quick cuts” after quenching consists of highly alloyed martensite containing 0.3-0.4% C, undissolved excess carbides and retained austenite. The higher the quenching temperature, the lower the position of points Mn, Mk and the more retained austenite.
In R18 steel there is approximately 25-30% retained austenite, in R6M5 steel - 28-34%. To reduce austenite, cold treatment can be done, but as a rule this is not required.
After quenching, tempering follows at 550 - 570°C, causing the transformation of retained austenite into martensite and dispersion hardening due to the partial decomposition of martensite and the release of dispersed carbides of alloying elements. This is accompanied by an increase in hardness (secondary hardness).
During the holding process during tempering, carbides are released from the retained austenite, which reduces its alloying, and therefore, upon subsequent cooling, it undergoes a martensitic transformation (Mn ~ 150°C). During a single tempering process, only part of the retained austenite is transformed into martensite. In order for all the austenite to transform into martensite, two and three times tempering is used.
The holding time is usually 60 minutes. When assigning a regime, it is necessary to take into account the chemical properties of the elements and the frequency of carbide release depending on temperature. For example, the maximum hardness of R6M5 steel is obtained through 3-stage tempering. The first tempering is at a temperature of 350°C, the next two at a temperature of 560-570°C.
At a temperature of 350°C, cementite particles are released, evenly distributed in the steel. This contributes to the uniform release and distribution of special M6C carbides at a temperature of 560-570°C.
Processing of tool alloys
High, medium and low steel tempering are only suitable for heat treatment of alloys containing less than 0.7% carbon. For alloys with a higher carbon content (called tool alloys), other methods are used. Let's look at the main technologies:
- It is not recommended to temper high-speed tool alloys because they contain molybdenum, cobalt, tungsten, and vanadium. These elements are resistant to heat, so they do not change their physical and chemical properties during tempering heating. Instead of tempering, it is recommended to do multi-stage hardening: for this, the material is gradually heated to 800, 1050 and 1200 degrees - after which the alloy is sharply cooled in an oil environment.
- It is recommended to process conventional tool alloys in two stages. First, the material is hardened in molten salts at a temperature of 450-500 degrees. After this, the second stage is performed - double tempering at a temperature of 550-600 degrees (no more than 1 hour). Please note that when tool alloys are heated, the possibility of the occurrence of tempering ability of the second type is excluded.
Heat treatment of steel: heating and cooling
The operations of heat treatment of steel, the basis of which is heating to a certain temperature and holding at it, were discussed earlier. These include: tempering, solution treatment and annealing.
Next, we will consider steel heat treatment operations, which include both heating and controlled cooling with varying intensities - from cooling in still air to sudden cooling with water.
Steel normalization
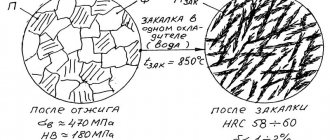
Steel normalization refers to the treatment of steel into a solid solution at a temperature no less than 55 ºC above the upper critical temperature, followed by cooling in air. The normalization temperature depends on the carbon content in the steel as shown in the figure. The purpose of normalization is usually to improve the grain structure and, in addition, to slightly strengthen the steel.
Figure - Simplified iron-carbon diagram. The shaded strip is the steel heating interval for normalization and hardening
When normalizing, the products are positioned so that when the cage cools after heating, air can circulate freely around each product. If air flow around heated products is obstructed, the heat treatment operation will be closer to annealing than normalizing. Accelerated cooling by fans or compressed air can produce a result more like hardening.
The microstructure that results from normalization is a mixture of ferrite and pearlite, usually with low residual stresses and almost no warping.
Some products, after normalization, are tempered to obtain a slight additional softening of the steel, as well as an additional reduction in residual stresses.
A homogeneous, normalized structure is usually easy to cut.
Steel hardening
To obtain higher strength and hardness than with normalization, it is necessary to apply a cooling rate of steel from the austenitization temperature that would ensure the transformation of austenite into bainite and martensite, and not into a ferrite-pearlite mixture. This operation is called hardening. Hardening consists of austenitizing the steel at the temperatures shown in Figure 1, and then cooling it quickly enough so that ferrite and pearlite do not have time to form.
Quenching media
The maximum achievable hardness of hardened steel depends almost exclusively on the carbon content. It is achieved by cooling at a rate equal to or higher than the critical cooling rate for a given alloy. Water, salt solutions, oil, water-polymer solutions and, in some cases, inert gases are used as a quenching medium.
Hardening steel in water and oil
Typically, water and salt solutions are used when hardening steel. Where possible, cheaper water is used. However, hardening, for example, high-carbon steels, requires the use of oil. When hardening steel products with complex shapes, oil is also often used to minimize warping and cracking. Cooling of steels with oil is almost always slower than with water.
Hardening of steel in solutions of organic polymers
Some organic polymers, when added to water, give it hardening properties similar to those of oil. The main advantage of these solutions is that they remove heat more slowly than water, but without the fire hazard associated with oil.
The disadvantage of polymer solutions is that they require strict control of concentration, temperature and mixing to achieve consistent hardening results.
The degree of quenching hardness in salt baths can vary widely and depends on the type of polymer, its concentration, bath temperature and the intensity of mixing of the solution during quenching.
Interrupted hardening of steel
In some cases, it is necessary to harden steel in water or saline solution to obtain high surface hardness of the product. However, cooling with water or a saline solution until the steel is completely hardened can lead to warping of the product or the formation of hardening cracks.
If there is no need to strengthen the steel over its entire cross-section, then so-called interrupted hardening is often used. In English it is also called “slack quenching”, weak or weakened quenching.
Interrupt quenching typically involves quenching in water for a specified time and then the workpiece is transferred to an oil bath to complete the transformation.
Hardening of carbon and low-alloy steels is always accompanied by tempering.
Tempering of hardened steel
Tempering is the process of heating hardened steel to a temperature below the lower critical temperature, followed by cooling to room temperature.
The purpose of tempering is to reduce internal stresses and reduce hardness and thereby obtain higher ductility than in the case of hardened products without tempering.
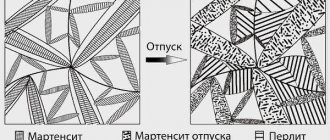
Tempering slightly modifies the structure of martensite and this change is used to “adjust” strength, hardness, toughness and other mechanical properties to specified values.
Carbon and low-alloy steels are tempered in the temperature range from 175 to 700 °C. The exposure time can vary from 30 minutes to several hours.
A longer tempering time at a given temperature or a higher temperature at a given exposure increases the degree of softening of the steel.
At the same temperature, martensite reduces its strength and hardness more significantly than pearlite, and the rate of change in the properties of steel during tempering depends on its chemical composition.
Tempering brittleness of steel
Some alloy steels may suffer from temper embrittlement when tempered at temperatures below 595°C. For such steels, they try to avoid being in the temperature range of temper brittleness or go through it at high speed.
How to release steel yourself
In order to temper steel at home in order to relieve internal stress, it is not necessary to know its grade - it is enough to heat it to a temperature no higher than 200 ºC and hold it in these conditions for at least an hour.
If you plan to temper a steel product to reduce hardness and increase toughness, then knowledge of the steel grade is necessary to determine the tempering temperature conditions. This is actually not as difficult a task as it might seem. In textbooks on heat treatment and on Internet sites there are enough tables with lists of products and the grades of steel from which they are made, and often even with the temperature conditions for their quenching and tempering (see table above).
To heat your part, you can use almost any heat source: from the oven to a gas burner or a homemade forge. An important point is the heating temperature. In principle, it can be determined by color tables of tarnish appearing on hot metal, which are also easy to find on the Internet.
This is an old proven method, known since ancient times, but it requires some experience, since its main disadvantages are the subjectivity of color perception and its dependence on external lighting. For a beginner, the best solution would be to use a stove thermostat or a regular multimeter with a thermocouple.
Has anyone ever used a multimeter with a thermocouple to measure the tempering temperature? How accurate is this device and how do its readings correspond to the color of the tarnish? If anyone has such experience, please write your opinion in the comments.
Tempering brittleness of steel
Metal tempering is the technological process of heat treatment of a hardened steel alloy.
It makes it possible to complete phase transformations in the microstructure (martensite), which acquires the most stable state.
The fact is that during the hardening process, internal stresses arise in the metal - axial, radial, tangential.
To eliminate their negative consequences such as fragility and low ductility, products are heated in ovens at different temperatures (from 250 °C to 650 °C), kept for a specified time (from 15 minutes to 1.5 hours), and then slowly cooled.
The complex of these measures leads to the release of excess carbon, restructuring and ordering of the metal structure, and the elimination of defects in its crystalline structure.
The processed materials acquire a given set of mechanical properties, among which the main ones are an increase in ductility and a decrease in fragility while maintaining a sufficient level of strength.
Types of steel tempering
- Short.
- Average.
- High.
Low holiday concept.
To reduce internal stresses, low tempering of steel is usually carried out by heating to 250 °C for 1 to 2.5 hours.
During the process of diffusion, some of the excess carbon is released from the metal, from which carbide particles are formed in the form of plates and rods.
The nonequilibrium structure of quenched martensite transforms into equilibrium tempered martensite.
This achieves stabilization of product dimensions , increases viscosity and strength, and hardness indicators practically do not change.
Iron-carbon and low-alloy steels are subjected to low-temperature tempering to produce cutting and measuring tools that do not experience dynamic loads. It is mainly performed for steels hardened by high-frequency currents, as well as for alloys whose surface was previously saturated with carbon and nitrogen.
Features of an average vacation.
It is carried out at temperatures from 350 °C to 500 °C and provides high elasticity and relaxation resistance.
All excess carbon is released from the steel, and the carbide turns into cementite.
Martensite has already completely decomposed, and the restructuring of the metal structure (polygonization) and its improvement (recrystallization) have not yet begun.
The new combination is called troostomartensite and is characterized by accelerated diffusion processes. In this case, the crystal lattice of the alloy turns into a cubic lattice, and internal stresses decrease even more.
The metal is cooled in water, which also increases the endurance limit. Medium-temperature tempering is necessary in the production of elastic parts: springs, impact tools and springs.
High release technology.
At temperatures above 500 °C, structural transformations occur in carbon alloys, which are no longer phase transformations.
The configuration and dimensions of crystal particles undergo changes, their grains become larger, and their shape tends to be equiaxed.
Complex heat treatment, including hardening and high tempering of steel, is called improvement in materials science, and the crystalline structure of the metal after this is called sorbitol tempering.
It is considered the most effective, since an ideal combination of viscosity, ductility and strength of the alloy is achieved. However, the hardness decreases somewhat, so there is no hope for improved wear resistance.
The duration of high tempering varies from 1 to 6 hours and depends on the sizes of gears, bearings, crankshafts, bushings, bolts and screws made of structural and medium carbon steels. During operation, these products absorb shock loads and work under compression, tension and bending, and special requirements are placed on their strength, endurance, fluidity and impact strength.
Process description
Steel tempering (STE) is a type of heat treatment in which the metal is gradually heated and then cooled. In most cases, the tempering procedure is performed at the final stage immediately after hardening. OS can be performed both before and after the formation of a part from a semi-finished steel product. Allows you to eliminate internal stresses inside the metal, which negatively affect its physical structure and properties.
Internal stresses at the chemical level are violations of the crystalline structure of the metal. Because of them, there is an uneven distribution of carbon and alloying additives throughout the metal alloy. Vacation allows you to redistribute these elements more evenly. This improves the physical and chemical properties of the material (ductility, strength, shape retention, chemical inertness). Heating is carried out using special furnaces in a protective environment (oil, saltpeter or alkaline baths). The method of cooling parts after heating is air (usually) or liquid (rarely).
The quality of steel tempering depends on the following physical parameters of the thermal procedure:
- Heating temperature. OS can be performed at temperatures from 100 to 700 degrees, and the higher the heating temperature, the higher the quality of processing. This dependence is explained by the fact that at higher temperatures a deeper change in the structure of the crystal lattice occurs. Mainly due to the processes of polygonization and recrystallization.
- Heating duration. OSes typically last between 1 and 3 hours, although longer formats exist. All main processes in the material take place in the first 20-40 minutes. Additional exposure is needed for uniform distribution of carbon atoms, iron, and alloying additives throughout the entire thickness of the material.
- Cooling speed. The rule here is extremely simple - the slower the cooling, the higher the quality of the material. To slow down cooling, metallurgists use various tricks and tricks. The main trick is to place the material in an oil, saltpeter or alkaline environment, which slows down the cooling of the material. Theoretically, cooling can be performed without the use of liquid media, but the cooling rate will be high, which will negatively affect the quality of the OS.
Steel tempering: types and purposes
Tempering is the final stage of heat treatment of steel. Performed after hardening. The quality and service life of the part depends on it.
The task is to heat the steel billet to a temperature below the critical level, after which the value is maintained for a certain period of time and slowly (or quickly, depending on the specifics of the technical process) tempered to the desired value.
The following actions are performed:
- Possible stress in the steel workpiece is reduced or completely eliminated.
- The viscosity of the metal increases to the value required by operating conditions.
- The hardness of the workpiece decreases, this is important for its processing.
The main processes during the operation are: decomposition of martensite, subsequent polygonization, recrystallization.
The product is heated in an oven from 150-250 to 370-650 ºC, the value is controlled smoothly, sudden changes in indicators are unacceptable.
Short
The procedure is carried out taking into account heating in the oven to 150-250 ºC. Next, a long exposure is carried out, taking into account the temperature value; the final stage is cooling the workpiece in the open air.
When a steel billet is seasoned, martensite takes the form of tempering within a specified temperature range. The previously formed stress in the structure will be relieved, and the residual austenite will transform into martensite of a similar shape. If the steps are carried out correctly, the strength of the part is achieved, and it can be easily processed to obtain the required shape and dimensions.
Upon completion of the operation, the metal remains hard, but in some cases, the indicator increases. The result is achieved due to the decomposition of retained austenite. In parallel with the preservation of hardness, the brittleness of hardening is localized.
This type of operation is used in the manufacture of various products and cutting tools, provided that high hardness of the structure is ensured. Thanks to the transformation of martensite, the dimensions of the workpiece are stabilized.
This is relevant provided that the parameters of the measuring instrument, in the manufacturing process of which tool steel is used, are observed. When making an instrument, this type of operation is performed.
Average
A temperature of 300-500 ºC is required. The hardness at the last stage rapidly decreases, but the viscosity value increases. It is possible to obtain tempered troostite, the hardness of the metal increases to 43 HRC.
It is used in the production of springs, leaf springs, special technological tools, which are characterized by high strength and elasticity.
In this case, the hardness is set at an average level, this will allow the workpiece to be processed and given the desired characteristics.
High
It is carried out taking into account the temperature regime of 500-600 ºC. The main purpose is to obtain maximum toughness with an optimal combination of strength and elasticity of the steel structure.
In practice, this is used in the manufacturing process of parts made from structural grades. While performing work, they are exposed to high voltage.
This is relevant when the metal structure is exposed to shock loads during casting.
During the manufacture of parts designed for the use of various types of mechanisms and machine tools, it is customary to use heat treatment. The essence is to harden the workpiece with further high tempering.
It is carried out taking into account the preservation of temperature, which ensures the production of sorbitol, excellent ductility and strength of the metal. The processing process is called “improving the characteristics of the metal.”
Heating in the metal may also be provided. It is performed exclusively in furnaces used in production when carrying out other methods of processing the workpiece. It will be necessary to ensure uniform temperature throughout the entire stage, while simultaneously accurately monitoring the condition of the metal.
Tempering brittleness
In parallel with the increase in the tempering temperature, the impact strength increases; cooling does not affect the characteristics. For certain steel grades, a decrease in this indicator is typical; the defect is called “temper brittleness.”
There are two types of phenomena, each of which is distinguished by the specifics of its formation and subsequent result. Pay attention to the features of each of them; the development of the technological process for creating the workpiece depends on this.
Type 1 temper brittleness
Occurs when the temperature range passes 300 ºC. This is not related to the cooling parameters of the workpiece at the final stage of processing.
This manifestation is caused by the difference in the levels of martensite transformation in the workpiece being created.
The measured value of fragility is irreversible; even if this element is heated again, it will not appear, therefore, the structure remains in a stable state.
Tempering brittleness 2nd kind
The phenomenon manifests itself in the structure of alloy steels when they are slowly cooled. The temperature is set to 450-650 ºC. When a high tempering takes place during the casting of a workpiece, the separation of dispersed inclusions of carbides is noted along the boundaries of the metal. Upon examination, the border zone is united due to the presence of alloying components.
When smooth cooling occurs, diffusion is formed; it manifests itself more sharply towards the grain boundaries. Parts of the structure in the border region are enriched in phosphorus. This manifestation will reduce the level of toughness as well as strength.
It is noted as a reversible process; with secondary heating, smooth cooling to the desired value, if an interval dangerous for the indicators is set, the defect has every chance of reoccurring.
Steels that tend to develop this type of brittleness in their structure cannot be heated to 650 ºC.
A decision is made to conduct a vacation of one type or another, depending on the characteristics of the workpiece, performance indicators, as well as the needs of the production process.
It is important to maintain the temperature and subsequently carry out natural cooling of the workpiece, which will allow you to achieve an impressive result.
There is nothing complicated in the process if you work out a map of technological operations in advance.
AVERAGE HOLIDAY
Average tempering is carried out at temperatures of 350–400 °C. In this case, all excess carbon is released from martensite with the formation of cementite particles. The tetragonality (degree of tetragonality) of the iron lattice decreases, it becomes cubic. As a result, ferrite remains instead of martensite. Such a ferrite-cementite mixture is called tempering troostite, and the process leading to such changes is called medium-temperature tempering. With medium tempering, the dislocation density decreases and the internal stresses in the steel decrease.
Medium tempering is used for heat treatment of elastic parts: leaf springs, springs, etc.
What steel tempering technologies exist?
When metals are hardened, internal stress is generated. If it is not eliminated, the finished product will have a high fragility rating. Plasticity will be significantly below normal. To eliminate these problems, steel tempering is used. It is one of several heat treatment processes for metals.
What is a vacation?
Metal tempering is a thermal process that is used for all hardened parts. Many novice craftsmen do not understand how important the set of heat treatment stages is for a material.
Heat treatment of metals improves the characteristics of a metal part. During such processing, the structure of the steel changes. Because of this, individual properties of the material deteriorate or improve.
This heat treatment allows you to relieve the internal stress that forms after hardening the steel. If this is not done, the material will be fragile and will not withstand serious loads. In addition to relieving internal stress, this process increases the hardness of the steel. This is an important feature in the manufacture of tools and parts for industrial equipment.
The temperature regime is selected depending on what grade of material will be processed. Based on this, the metal can be cooled in different solutions:
- in containers filled with molten alkali;
- in baths filled with saltpeter;
- in containers with oil or water.
In production, metal parts are cooled in ovens. In this case, a forced ventilation system is installed on the equipment. REMOVAL OF STEEL THE SIMPLE METHOD
Kinds
The steel tempering temperature is considered the most important parameter when carrying out this technological process. There are three types of tempering heat treatment. Features of the technological process depend on the type of heat treatment.
Heat treatment of tool alloys
Tool alloys or high-speed metals used for the manufacture of wear-resistant tools must be heat treated. As temperatures increase, their ductility does not increase and strength does not decrease.
To improve the characteristics of tool alloys, alloying additives are added to their composition - tungsten, molybdenum, vanadium or cobalt. Next, the workpieces are hardened at a temperature of 1200 degrees.
Tempering is considered one of the key stages of heat treatment. It allows you to relieve internal stress and increase the strength of the metal. It is important to choose the correct temperature regime and cooling rate of the workpiece. For cooling, containers with various solutions are used.
Is it possible to temper steel at home?
Most often, heat treatment applies to various simple parts, household utensils - knives, forks, metal cups, car parts, and so on. However, home metallurgy has many limitations that the common man may not be aware of. Let's consider the main problems that a person may encounter during a steel holiday at home:
- Most home ovens cannot reach high temperatures. Therefore, at home you can only make a low or medium vacation. Theoretically, you can try to re-equip or “strengthen” your stove to increase the heating temperature, but this will be difficult for a person without experience.
- To carry out heat treatment, it is necessary to use a protective medium (oil, alkali, saltpeter). But each substance has its own temperature characteristics. A simple example: saltpeter-based compounds can explode when heated to high temperatures, which can be dangerous to the life and health of the home metallurgist.
- Tempering without the use of a protective environment can be fatal to the metal itself. The fact is that without the use of a protective environment, the metal will cool quickly, which can affect the quality of the steel (increased brittleness, bending, plastic deformation, rust).
- Also, do not forget about low-temperature brittleness of the first type (from 250 to 300 degrees). If the temperature is incorrect, the quality of the metal can be seriously affected, up to the complete destruction of the alloy.