Mechanical properties of steel:
- Strength is the ability to withstand external loads without collapsing. This property is characterized by tensile strength (mechanical stress at which a steel product breaks) and yield strength (mechanical stress at which the product continues to elongate without any load).
- Hardness is the ability to resist penetration of solids. Measured by the Brinell and Vickers methods. The Brinell method involves determining strength using the load per indentation area in the process of pressing a steel ball, and the Vickers method uses a diamond pyramid.
- Plasticity is the ability to change shape after the action of a load and retain its shape after the load is removed. Characterized by relative tensile elongation and bend angle.
- Impact strength is the ability to withstand dynamic loads. You can estimate the work required to destroy the sample, divided by the cross-sectional area.
Tensile strength
PP - we will use this abbreviation, and we can also talk about the official combination “temporary resistance” - this is the maximum mechanical force that can be applied to an object before its destruction begins. In this case, we are not talking about chemical effects, but we mean that heating, unfavorable climatic conditions, and a certain environment can either improve the properties of the metal (as well as wood, plastic) or worsen it.
No engineer uses extreme values when designing, because it is necessary to leave a permissible error - for environmental factors, for the duration of operation. We told you what is called tensile strength, now let’s move on to the specifics of the definition.
Chemical properties of steel:
- Heat resistance - the ability not to oxidize at high temperatures, not to form scale.
- Heat resistance - the ability to maintain strength at high temperatures.
- Oxidability - the ability to combine with oxygen. The higher the temperature of the metal, the higher the oxidation. If low carbon steel is exposed to moisture or humid air, it will oxidize, forming iron oxide - rust.
- Corrosion resistance is the ability not to oxidize, not to enter into a chemical reaction with substances that surround the metal.
How is the strength test performed?
Initially there were no special events. People took an item, used it, and as soon as it broke, they analyzed the breakdown and reduced the load on a similar product. Now the procedure is much more complicated, however, until now the most objective way to find out PP is the empirical way, that is, experiments and experiments.
All tests are carried out under special conditions with a large amount of precise equipment that records the condition and characteristics of the experimental material. Usually it is fixed and experiences various influences - tension, compression. They are performed by instruments with high precision - every thousandth of a newton of the applied force is noted. At the same time, each deformation is recorded as it occurs. Another method is not laboratory, but computational. But usually mathematical analysis is used in conjunction with testing.
Definition of the term
The sample is stretched on a testing machine. In this case, first it lengthens in size, and the cross section becomes narrower, and then a neck is formed - the place where the thinnest diameter is, this is where the workpiece will rupture. This is true for ductile alloys, while brittle alloys, such as cast iron and hard steel, stretch very slightly without necking. Let's take a closer look at the video:
Technological properties of steel:
- Weldability is the ability to form a high-quality welded joint.
- Malleability is the ability to change shape under the influence of an external force.
- Machinability is the ability to be machined mechanically using a cutting tool.
- Fluidity is the ability of a metal in a molten state to fill small spaces.
Carbon content is of great importance for the quality of steel. The higher the content of this element in the metal, the higher the hardness and wear resistance. However, high carbon content has a detrimental effect on the ductility and weldability of the metal.
Low-carbon steels of ordinary quality
From the group of low-carbon steels of ordinary quality produced by the metallurgical industry in accordance with GOST 380 - 88, steel grade St3 is widely used in construction.
Steel grade St3 is produced boiling (ST3kp), semi-quiet (St3ps) and calm (St3sp).
Depending on the purpose, steel is supplied in the following three groups, which indicate the properties by which steel is standardized:
A - by mechanical properties;
B - by chemical composition;
B - according to mechanical properties and chemical composition
Since it is necessary to ensure strength and weldability for load-bearing building structures, as well as adequate resistance to brittle fracture and dynamic impacts, steel for these structures is ordered according to group B, i.e., with a guarantee of mechanical properties and chemical composition.
Steel grade St3 contains 0.14 – 0.22% carbon.
According to GOST 380 - 88, steel marking is done as follows: first put the corresponding letter designation of the steel group, then the grade, then the deoxidation method and finally the category; for example, steel of group B (supplied according to mechanical properties and chemical composition) grade St3 is semi-quiet, category 5 is designated VSt3ps5.
The category indicates which mechanical properties of steel are preserved at temperatures of -20 and +20 degrees Celsius. Ordinary quality steels are divided into 5 categories. The table of standardized indicators by category is given in GOST 535-88.
Color coding
Color marking of steels is used to indicate rolled products. This is convenient when storing materials in warehouses and transporting them. Steels are marked with marks in the form of dots or stripes made with indelible paint. The color of the designations is selected from the table according to the purpose of the steel. In this case, the steel group and the degree of its deoxidation are not taken into account.
Example of steel color marking
Classification by structure
The structure of steel refers to the internal structure of the metal, which can vary significantly depending on heat treatment conditions and mechanical influences. The shape and size of grains depend on the composition and ratio of alloying additives and production technology.
The basis of steel grains is a crystal lattice of iron, which includes atoms of impurities - carbon, metals. Carbon can form solid solutions in the crystal lattice, or it can create chemical compounds, carbides, with iron.
Metal additives exist in the form of solutions, and many of them affect the state of the carbon solution.
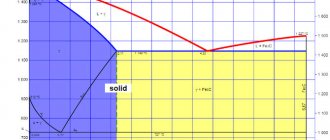
The structure of steel changes with temperature changes. These changes are called phases. Each phase exists in a certain temperature range, but alloying additives can significantly shift the boundaries of the transition of one phase to another.
The following are the main phases of the state of the metal:
- Austenite. Carbon atoms are located inside the iron crystal lattice. This phase exists in the range of 1400-700 °C. If the composition contains from 8 to 10% nickel, the austenite phase can persist at room temperature.
- Ferrite. Solid solution of carbon in iron.
- Martensite. Supersaturated carbon solution. This phase is characteristic of hardened steel.
- Bainite. The phase is formed by rapid cooling of austenite to a temperature of 200-500 °C. Characterized by a mixture of ferrite and iron carbide.
- Perlite. Equilibrium mixture of ferrite and carbide. It is formed when austenite is slowly cooled to a temperature of 727 °C.
The phases of the metal structure characterize its physical properties, depending on which the class of steel is determined - structural, foundry, and so on.
Marking of steels according to Russian standards
Marking steel according to Russian standards makes it possible to determine the composition of the metal and, in part, whether it belongs to a certain type.
If the presence of carbon in steel is more than 1%, its amount is not indicated in the marking. The steel grade includes letter designations of alloying additives indicating their quantity in tenths and hundredths of a percent, but if the component content is less than 1.5%, then only the letter designation is present in the marking.
Read also: Sharpen a meat grinder knife with your own hands
In addition to the chemical composition, the marking contains symbols characterizing the purpose of the steel and the degree of its quality.
Types of steels and their properties
Steel refers to iron alloys containing carbon and other chemical elements. In this case, the carbon content should vary from 0.1% to 2.14%, and iron should not be less than 45%. Steels can be ordinary carbon, alloyed and high alloyed. The last two types of alloys contain alloying elements, due to which the steel acquires increased strength.
Under the influence of carbon, iron acquires improved physical characteristics - greater strength, strength, and reduced ductility. The properties of steel are influenced by alloying substances added to the alloy, enhancing the above parameters.
Steels can be classified according to various characteristics. Let's talk about the mentioned classes, which are based on the chemical properties of the metal:
1. Carbon steels.
This group is represented by alloys of iron and carbon in the absence of additional alloying elements. They are intended for solving design and instrumental problems.
Depending on the carbon content in the composition, the entire group can be divided into subgroups:
- low-carbon steels (containing less than 0.25% carbon);
- medium-carbon (with a content of 0.25–0.6% carbon);
- high-carbon (containing up to 2% carbon).
The distinctive properties of the steel alloy are high strength and hardness, as well as the minimal presence of impurities.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
2. Alloy steels.
Alloying elements are added to these alloys in order to increase their strength. They are divided into subgroups in accordance with the quality and quantity of alloying substances present in them. They relate to:
- low-alloyed (if the content of alloying substances is less than 4%);
- moderately doped (substance concentration varies from 4% to 11%);
- highly alloyed (with a content of more than 11% alloying substances).
Alloying elements can be chromium (Cr), nickel (Ni), molybdenum (Mo). The properties of alloy steels, obtained by the interaction of the above elements with iron and carbon, are high wear resistance and strength.
There is also a classification of steels according to their structure:
1. Austenitic steel.
In austenitic steels, during the crystallization process, a single-phase austenitic structure γ-Fe is formed, which is characterized by a face-centered crystal lattice, which is preserved when cooled to cryogenic temperatures.
This type of alloy is represented by the following subtypes:
- corrosion resistant;
- heat-resistant;
- heat resistant;
- cold-resistant austenitic steels.
Otherwise, the alloys included in this group are called austenitic steels.
2. Ferritic steel.
This name is given to steel, which contains alloyed ferrite with the inclusion of carbides. To obtain it (ferritic class), iron is combined with a small amount of carbon and a significant concentration of alloying substances (vanadium, silicon).
3. Martensitic steel.
Martensite is a needle-like microstructure that occurs in a number of pure metals as well as in hardened metal alloys. The structure of martensitic steels is dominated by martensite. In addition to iron, their composition is characterized by an insignificant carbon content (approximately 0.2%) and a noticeable concentration of chromium (within 11–17%). Nickel, vanadium or molybdenum can also be found in alloys. Among the properties of martensitic steels are resistance to alkalis, low ductility, increased heat resistance, and the ability to self-harden.
4. Bainitic steel.
Bainite is a steel structure formed during the intermediate transformation of austenite, for which reason it is also called an intermediate structure. Alloys of the bainite class are characterized by the presence of alloying substances and low carbon concentration.
5. Pearlitic steel.
Pearlitic steels can be divided into subgroups represented by:
- hypoeutectoid alloys (carbon content in which does not exceed 0.8%);
- eutectoid (in which 0.8% carbon is present);
- hypereutectoid (with carbon content in the range of 0.8–2%).
They are characterized by a relatively low concentration of alloying substances.