Effect of nitrogen on metal properties
Solubility of nitrogen in steel and its effect on the properties of steel. The solubility of nitrogen in iron can be described by the equations
- 2 [N] = N2 (g);
- KN = pN2/[N] 2
Consequently, the solubility of nitrogen, in accordance with the Sieverts rule, is proportional to the square root of its pressure. Since KN changes with temperature and is different for different phase states, the solubility of nitrogen depends on temperature and changes abruptly during allotropic transformations and melting.
The research results, shown in the form of an isobar in Fig. 1 show that the solubility of nitrogen in steel decreases sharply during crystallization and during the transformation of y-iron into a-iron.
Other elements affect the nitrogen solubility of liquid steel. This influence is characterized by interaction parameters. Their sign and magnitude show that, in order of increasing influence, the solubility of nitrogen increases with molybdenum, sulfur, manganese, aluminum, chromium, vanadium, and titanium. Reduce the solubility of nitrogen Ni, P, C.
| Figure 1. Solubility of N2 in iron at a partial nitrogen pressure of 10.223 kPa/m2 (760 mm tst) |
A decrease in the solubility of nitrogen in steel during crystallization and during the transformation of y-iron into a-iron is the main reason for the influence of nitrogen on the properties of steel.
In the absence of elements (Ti, Al, Zr, V) in steel that form nitrides at high temperatures, after the formation of a-iron, nitrogen begins to be released from the solution in the form of inclusions of iron nitrides (Fe2N, Fe4N, Fe8N). This precipitation can continue for a long time after cooling and, since it occurs mainly at low temperatures, the released inclusions are very dispersed. The order of their size is 10 -3 microns.
Dispersed inclusions of iron nitrides are located along crystallographic planes and, preventing the movement of dislocations, cause embrittlement of the metal. As a result, the impact strength decreases and the relative contraction and elongation decrease while the hardness and strength properties increase.
Like the release of iron nitrides, the drop in impact strength increases with the time of storage or operation of steel products, reaching a minimum after 20-40 days. Therefore, the described phenomenon is called aging.
Nitrogen in steel causes aging, which can be accelerated artificially if the hardened iron or steel is subjected to cold plastic deformation, which increases the rate of decomposition of the solid solution and the release of iron nitrides.
As a result of aging, impact strength can decrease by 4-6 times. Therefore, the tendency to aging is a significant defect of steel. It is typical for low-carbon steel that has not been deoxidized with aluminum or vanadium.
Adding elements to steel that bind nitrogen in steel into nitrides at high temperatures eliminates the tendency of steel to age. These elements include: aluminum, which forms nitrides mainly during solidification and in solid metal up to the temperature at which y-iron transforms into a-iron; vanadium and zirconium, forming nitrides during crystallization; titanium, which forms nitrides in liquid steel and during crystallization.
The most widely used is aluminum, which is also widely used as a deoxidizer. At normal concentrations of aluminum and nitrogen in steel, it forms nitrides in the solid metal. But the sizes of inclusions of these nitrides, released at higher temperatures, are 2-3 orders of magnitude larger than the sizes of inclusions of iron nitrides. Therefore, they do not have such an effect on the movement of dislocations and do not cause aging.
Consequently, the metal deoxidized by aluminum has a low nitrogen content in the steel, and it is not prone to aging. However, in steel deoxidized with aluminum, a decrease in impact toughness can be observed. This manifests itself at a high content of nitrogen and aluminum (for example, 0.01% N and 0.2% Al), when an intergranular fracture is formed in the metal, passing along the grain boundaries of primary austenite. The formation of such a fracture is caused by a weakening of the bond between grains due to the precipitation of aluminum nitride inclusions along their boundaries and it indicates a deterioration in the properties of steel.
Steel nitriding: purpose, technology and process types
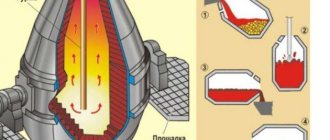
Nitriding, during which the surface layer of a steel product is saturated with nitrogen, began to be used on an industrial scale relatively recently. This processing method, proposed for use by Academician N.P. Chizhevsky, allows you to improve many characteristics of products made from steel alloys.
Ion-vacuum nitriding workshop
The essence of technology
Nitriding of steel, when compared with such a popular method of processing this metal as carburization, has a number of significant advantages. That is why this technology began to be used as the main way to improve the quality characteristics of steel.
When nitriding, the steel product is not subjected to significant thermal effects, while the hardness of its surface layer increases significantly. It is important that the dimensions of the nitrided parts do not change.
This allows this processing method to be used for steel products that have already been hardened with high tempering and ground to the required geometric parameters.
Once nitriding, or nitriding as the process is often called, has been completed, the steel can be immediately subjected to polishing or other finishing methods.
Scheme of a glow discharge nitriding installation
Nitriding of steel involves heating the metal in an environment characterized by a high ammonia content. As a result of this treatment, the following changes occur with the surface layer of the metal, saturated with nitrogen.
- Due to the fact that the hardness of the surface layer of steel increases, the wear resistance of the part improves.
- The fatigue strength of the product increases.
- The surface of the product becomes resistant to corrosion. This stability is maintained when steel comes into contact with water, moist air and air-steam environment.
Microstructure of a high-quality nitrided layer of steel grade 38Х2МУА
Nitriding allows you to obtain more stable steel hardness indicators than carburization.
Thus, the surface layer of a product that has been subjected to nitriding retains its hardness even when heated to a temperature of 550–600°, while after carburization, the hardness of the surface layer may begin to decrease when the product is heated above 225°. The strength characteristics of the surface layer of steel after nitriding are 1.5–2 times higher than after hardening or carburization.
How does the nitriding process proceed?
Metal parts are placed in a hermetically sealed muffle, which is then installed in a furnace for nitriding. In the furnace, the muffle with the part is heated to a temperature that is usually in the range of 500–600°, and then maintained for some time at this temperature.
Vacuum heat treatment furnace with gas nitriding system
To create the working environment inside the muffle necessary for nitriding to take place, ammonia is supplied to it under pressure. When heated, ammonia begins to decompose into its constituent elements; this process is described by the following chemical formula:
2NH3 → 6H + 2N.
Atomic nitrogen, released during this reaction, begins to diffuse into the metal from which the workpiece is made, which leads to the formation of nitrides characterized by high hardness on its surface. To consolidate the result and prevent the surface of the part from oxidizing, the muffle, along with the product and the ammonia that continues to remain in it, is slowly cooled together with the nitriding furnace.
The nitride layer formed on the metal surface during the nitriding process can have a thickness in the range of 0.3–0.6 mm. This is quite enough to provide the product with the required strength characteristics. Steel processed using this technology does not need to be subjected to any additional processing methods.
Classification of nitriding processes
The processes occurring in the surface layer of a steel product during its nitriding are quite complex, but have already been well studied by specialists in the metallurgical industry. As a result of such processes, the following phases are formed in the structure of the processed metal:
- Fe3N solid solution, characterized by a nitrogen content in the range of 8–11.2%;
- Fe4N solid solution, which contains 5.7–6.1% nitrogen;
- a nitrogen solution formed in α-iron.
An additional α-phase in the metal structure is formed when the nitriding temperature begins to exceed 591°. At the moment when the degree of saturation of a given phase with nitrogen reaches its maximum, a new phase is formed in the metal structure. Eutectoid decomposition in the metal structure occurs when the degree of its saturation with nitrogen reaches a level of 2.35%.
Valves of high-tech internal combustion engines must undergo a nitriding process
Factors influencing nitridation
The main factors that influence nitriding are:
- the temperature at which such a technological operation is performed;
- gas pressure supplied to the muffle;
- duration of exposure of the part in the oven.
The efficiency of this process is also influenced by the degree of ammonia dissociation, which, as a rule, is in the range of 15–45%.
As the nitriding temperature increases, the hardness of the formed layer decreases, but the process of diffusion of nitrogen into the metal structure accelerates.
A decrease in the hardness of the surface layer of a metal during its nitriding occurs due to the coagulation of nitrides of alloying elements included in its composition.
The influence of temperature and alloying elements on the formation of a nitrided layer
To speed up the nitriding process and increase its efficiency, a two-stage scheme is used. The first stage of nitriding when using this scheme is performed at a temperature not exceeding 525°. This makes it possible to impart high hardness to the surface layer of the steel product.
To perform the second stage of the procedure, the part is heated to a temperature of 600–620°, while the depth of the nitrided layer reaches the required values, and the process itself is almost doubled.
The hardness of the surface layer of a steel product processed using this technology is no lower than a similar parameter for products processed using a single-stage method.
Types of Nitrided Steels
Both carbon and alloy steels, characterized by a carbon content in the range of 0.3–0.5%, can be processed using nitriding technology.
The maximum effect when using such a technological operation can be achieved if steels are subjected to it, the chemical composition of which includes alloying elements that form hard and heat-resistant nitrides. Such elements, in particular, include molybdenum, aluminum, chromium and other metals with similar characteristics.
Steels containing molybdenum are not subject to such a negative phenomenon as temper brittleness, which occurs when a steel product cools slowly. After nitriding, steels of various grades acquire the following hardness:
Hardness of steels after nitriding
Alloying elements found in the chemical composition of steel increase the hardness of the nitrided layer, but at the same time reduce its thickness. The thickness of the nitrided layer is most actively influenced by chemical elements such as tungsten, molybdenum, chromium and nickel.
Depending on the scope of application of the product that is subjected to the nitriding procedure, as well as on its operating conditions, it is recommended to use certain grades of steel to carry out such a technological operation. So, in accordance with the technological problem that needs to be solved, experts advise using products made from the following steel grades for nitriding. 38Х2МУА
This is steel, which, after nitriding, has a high hardness of the outer surface.
Aluminum contained in the chemical composition of such steel reduces the deformation resistance of the product, but at the same time helps to increase the hardness and wear resistance of its outer surface.
The exclusion of aluminum from the chemical composition of steel makes it possible to create products of more complex configurations from it.
40X, 40HFA
These alloy steels are used for the manufacture of parts used in the machine tool industry.
30Х3М, 38ХГМ, 38ХНММА, 38ХН3МА
These steels are used for the production of products that are subjected to frequent cyclic bending loads during their operation.
30Х3МФ1
Products are made from this steel alloy, the accuracy of whose geometric parameters is subject to high demands. To impart higher hardness to parts made of this steel (these are mainly parts of fuel equipment), silicon can be added to its chemical composition.
Characteristics of some steels after nitriding
Technological scheme of nitriding
To perform traditional gas nitriding, innovative plasma nitriding or ion nitriding, the workpiece is subjected to a series of technological operations.
Preparatory heat treatment
This processing consists of hardening the product and its high tempering.
Hardening as part of this procedure is carried out at a temperature of about 940°, while the workpiece is cooled in oil or water.
The subsequent tempering after hardening, which takes place at a temperature of 600–700°, allows the metal being processed to be given a hardness at which it can be easily cut.
https://www.youtube.com/watch?v=4Du8XZJu_ic
Heat treatment modes before nitriding
Mechanical restoration
This operation ends with its grinding, which allows the geometric parameters of the part to be brought to the required values.
Protection of areas of the product that do not require nitriding
Such protection is carried out by applying a thin layer (no more than 0.015 mm) of tin or liquid glass. Electrolysis technology is used for this. A film of these materials that forms on the surface of the product does not allow nitrogen to penetrate into its internal structure.
Carrying out the nitriding itself
The prepared product is processed in a gas environment.
Recommended steel nitriding modes
Finishing
This stage is necessary in order to bring the geometric and mechanical characteristics of the product to the required values.
The degree of change in the geometric parameters of the part when performing nitriding, as mentioned above, is very insignificant, and it depends on factors such as the thickness of the surface layer that is saturated with nitrogen; temperature regime of the procedure.
A more advanced technology – ion nitriding – can guarantee the almost complete absence of deformation of the processed product.
When performing ion plasma nitriding, steel products are exposed to less thermal influence, due to which their deformation is minimized.
Unlike innovative ion plasma nitriding, traditional one can be performed at temperatures reaching up to 700°. For this purpose, a replaceable muffle or a muffle built into the heating furnace can be used.
The use of a replaceable muffle, into which the parts to be processed are loaded in advance, before being installed in the furnace, can significantly speed up the nitriding process, but is not always an economically viable option (especially in cases where large-sized products are subjected to processing).
Punch weighing more than 230 kg, subjected to nitriding treatment
Nitrogen in steel. The influence of nitrogen on the properties of steel. Nitrogen in steel during its production process, page 7
At even higher levels, which can be achieved by adding nitrogen-rich ferrochrome, the steel tends to release nitrogen gas as it solidifies. Therefore, in castings that are completely free of bubbles, the nitrogen content can only be increased to 0.1 - 0.15%. This addition of nitrogen to semi-ferritic chromium steel causes a reduction, and in some circumstances, the complete disappearance of the ferritic structural component. Even in ferritic steels containing about 30% Cr, a partial transformation α→γ can be caused when heated to the appropriate temperature. The consequence of this formation of austenite is a reduced tendency for grain growth at high temperatures, which is especially significant during welding. In this way, shaped castings from ferritic chromium steel can be obtained with a fine-grained structure.
Catalytic gas nitriding
This type of chemical treatment involves creating a special atmosphere in the stove. Dissociated ammonia is pre-treated on a special catalytic element, which significantly increases the number of ionized radicals. Features of the technology include the following points:
- Preliminary preparation of ammonia makes it possible to increase the proportion of solid solution diffusion, which reduces the proportion of reaction chemical processes during the transition of the active substance from the environment to iron.
- Provides for the use of special equipment that provides the most favorable conditions for chemical processing.
Steel nitriding process
This method has been used for several decades and allows changing the properties of not only metals, but also titanium alloys. The high costs of installing equipment and preparing the environment determine the applicability of the technology to the production of critical parts that must have precise dimensions and increased wear resistance.
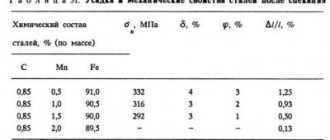
The influence of nitrogen on the properties of steel
In the absence of elements in steel that form nitrides at high temperatures (Ti, Al, Zr, V), after the formation of ?-Fe, nitrogen is released from the solution in the form of inclusions of iron nitrides (Fe2N, Fe4N, Fe8N). This release can last for a long time after cooling and, since it occurs mainly at low temperatures, the released inclusions are dispersed (about 10-3 microns in size). Dispersed inclusions of iron nitrides are located along crystallographic planes and, interfering with the movement of dislocations, cause excessive brittleness of the metal. The consequence of this is a decrease in impact strength and relative contraction, with a simultaneous increase in hardness and strength.
Like the release of iron nitrides, the decrease in impact strength increases with prolonged storage or operation of steel products, reaching a minimum after 20–40 days, which is why the described phenomenon is called aging. Aging can be accelerated artificially if hardened iron or steel is subjected to cold plastic deformation, which increases the rate of decomposition of the solid solution and the release of iron nitrides. As a result of aging, the impact strength can decrease by four to six times, so the tendency to age is a disadvantage of steel. It is characteristic of low-carbon steel that has not been deoxidized with aluminum or vanadium.
The effect of nitrogen on the mechanical properties of steel is depicted in Figure 3.
Figure 3 – Effect of nitrogen on the mechanical properties of steel
Adding elements to steel that bind nitrogen into nitrides at high temperatures eliminates the tendency of steel to age. Similar elements are the following:
- 1) aluminum, which forms nitrides mainly during solidification and in solid metal up to the temperature of transformation of ?-Fe into ?-Fe;
- 2) vanadium and zirconium, forming nitrides during crystallization;
- 3) titanium, which forms nitrides in liquid steel and during crystallization.
Aluminum has gained the greatest use, and is widely used as a deoxidizer. At ordinary concentrations of nitrogen and aluminum in solid metal, nitrides are formed. However, inclusions of these nitrides, released at a higher temperature, are two to three orders of magnitude larger in size than inclusions of iron nitrides, therefore they do not have such an effect on the movement of dislocations and do not cause aging.
Thus, calm steel, deoxidized by aluminum, is not prone to aging. However, in steel deoxidized with aluminum, a decrease in impact strength can be observed. This is expressed at a high content of nitrogen and aluminum (for example, 0.01% N and 0.2% Al), when an intergranular fracture is formed in the metal, passing along the grain boundaries of primary austenite. The formation of such a fracture is caused by a weakening of the bond between grains as a result of the precipitation of aluminum nitride inclusions along their boundaries, and it indicates a deterioration in the properties of the metal.
To summarize the above, excessive nitrogen content in steel leads to a decrease in yield strength and tensile strength, and it is also a significant cause of aging of low-carbon steels. Steel produced in electric furnaces contains 0.008-0.012% nitrogen. Since nitrogen is a difficult-to-remove impurity, its negative impact can be neutralized by introducing a nitride-forming element to obtain high-strength nitrides. In this case, first of all, an increase in the ductile properties of steels is achieved. However, to minimize the harmful effects of nitrogen, it is desirable to obtain steel with a content of this element of less than 0.004%.
Main types of nitriding
There are several technologies used to carry out nitriding of steel. Let's take the following list as an example:
- Ammonia-propane environment. Gas nitriding has become very widespread today. In this case, the mixture is represented by a combination of ammonia and propane, which are taken in a ratio of 1 to 1. As practice shows, gas nitriding when using such a medium requires heating to a temperature of 570 degrees Celsius and holding for 3 hours. The resulting layer of nitrides is characterized by a small thickness, but at the same time the wear resistance and hardness are much higher than when using classical technology. Nitriding of steel parts in this case makes it possible to increase the hardness of the metal surface to 600-1100 HV.
- Glow discharge is a technique that also involves the use of a nitrogen-containing environment. Its peculiarity lies in the connection of the nitrided parts to the cathode; the muffle acts as a positive charge. By connecting the cathode, it is possible to speed up the process several times.
- The liquid medium is used a little less frequently, but is also highly effective. An example is a technology that involves the use of a molten cyanide layer. Heating is carried out to a temperature of 600 degrees, the holding period is from 30 minutes to 3 hours.
Ionic nitriding
In industry, the gas medium has become most widespread due to the ability to process large batches at once.
The influence of nitrogen on the properties of steels
The harmful effect of nitrogen (N) is that the rather large, brittle non-metallic inclusions it forms - nitrides - worsen the properties of steel. A positive property of nitrogen is that it is capable of expanding the austenitic region of the phase diagram of steels. Nitrogen stabilizes the austenitic structure and partially replaces nickel in austenitic steels. Nitride-forming elements vanadium, niobium and titanium are added to low-alloy steels. When controlled by hot working and cooling, they form fine nitrides and carbonitrides, which significantly increase the strength of the steel.
Cr- improves mechanical properties, increases heat resistance, heat resistance, corrosion resistance, hardness.
Ni cold resistance, increases ductility and viscosity, electrical resistance
Manganese - increases yield strength
Si- (up to 2%) increases the yield strength
Tungsten and molybdenum – increase hardness and strength
Bibliography
- Luzgin V.P., Yavoisky V.I. Gases in steel and metal quality.-Moscow: Metallurgy, 1983.-230 p.
- Yavoisky V.I., Luzgin V.P., Vishkarev A.F. Steel oxidation and methods for its control. -Moscow: Metallurgy, 1970.-285 p.
- Yakovlev Yu.N.-Izv. ANSSSR. Metals, 1971, No. 4, pp. 51-54.
- Perevyazko A.T., Popov S.S. Oxidation of the jet when releasing alloyed metal from an electric furnace into a ladle //Steel, 1977, No. 12, pp. 1096-1098.
- Shishkin Yu.I., Yavoisky V.I., Nechkin Yu.M. Influence of converter slag on manganese waste during deoxidation // Izv. universities Ferrous metallurgy, 1977, No. 11, pp. 79-81.
- 6. Kostyanoy V.I., Yavoisky V.I., Zinkovsky I.V.-Izv. universities Ferrous metallurgy, 1977, No. 5, p. 32-34.
- Kobusch G., Junkers L., Kremer G. - Ferrous metals, 1963, No. 9, p. 13-19.
- Yavoisky V.I., Gorokhov L.S., Kapyrin V.S. and others. Factors influencing the secondary oxidation of steel at continuous casting machines // Steel, 1977, No. 3, p. 212.
- Rutes V.S., Askoldov V.Ya., Evteev D.P. etc. Continuous casting technology. -M.: Metallurgy. 1971, pp.29-40.
- Manokhin A.I., Nosochenko S.B., Matevosyan E.P. Structure and non-metallic inclusions of a continuous ingot stabilized with aluminum //Steel, 1970, No. 12, pp. 42-46.
- Kuptsov G.V., Yavoisky V.I., Gorokhov L.S. Formation of non-metallic inclusions during steel crystallization // Izv. universities Ferrous metallurgy, 1971, No. 9, pp. 54-57.
- Yavoisky V.I., Nechkin Yu.M., Zinkovsky I.V. – Research and improvement of steel production processes: scientific. tr./MISiS. - M.: Metallurgy, 1970, issue. 56, pp.96-109.
- Bigeev A.M. Steel metallurgy. – Chelyabinsk: Metallurgy, Chelyabinsk branch, 1988.-479 p.
© Perevorochaev N.M., 2011
- ← Influence of crystallizer swing parameters on the surface quality of continuously cast billets
- Increasing the productivity of six-strand continuous casters of PJSC Enakievo Metallurgical Plant. Achieved indicators. Development prospects →
Characteristics of the main structural classes of steels. The main ways to improve the quality of steel
Ferritic
Fe2O3 (ferrite structure) is formed with a low carbon content and a large amount of alloying element. Ferrite-forming elements Cr, Si, Mo, V, W, Zr. it is an alpha iron carbon interstitial solid solution
Used for non-critical parts
Pearlitic
(pearlite structure) – a mechanical mixture of ferrite and cementite plates
Austenitic
(austenite structure) is a solid solution of interstitial carbon gamma iron
Martensitic
(martensite structure) - observed in hardened alloys, supersaturated solid solution of carbon in alpha iron
Carbide or Ledeburite
(the structure consists of carbides of various Me) - a eutectic mixture of austenite and cementite, in the range 727-1147c
The quality of metal can be improved by reducing harmful impurities, gases, and non-metallic inclusions in it. To improve the quality of metal, treatment with synthetic slag, vacuum degassing of metal, electroslag remelting, vacuum-arc remelting, and metal remelting in electron-arc and plasma furnaces are used.
Vacuum degassing is carried out to reduce the content of gases in the metal due to a decrease in their solubility in liquid steel at reduced pressure and non-metallic inclusions.
Secondary oxidation of metal during casting
When casting on a continuous caster, intensive development of the process of secondary oxidation of the metal is possible, since interaction with atmospheric oxygen can occur on the way from the steel-pouring to the tundish and further on the way from the tundish to the crystallizer.
To study changes in the oxygen content in the metal during the period of continuous casting, special samples were taken simultaneously from the steel-pouring and tundish ladles, as well as from the crystallizer. The results of determining the oxygen content in selected samples from six melts of 09G2S steel are presented in Figure 2.
The data obtained show that in the sections steel-pouring - tundish, tundish - crystallizer, the metal is saturated with oxygen, i.e. the process of secondary oxidation occurs. The total increase in oxygen content averages 21 ppm.
During the research period, quartz protective tubes and submersible glasses without joint seals were used during casting.
Rice. 2
— Oxygen content in the metal at various technological stages of production (the numbers at the dots are the number of melts): s.k. – steel-pouring ladle; p.k – tundish; kr – crystallizer
Electroslag remelting and Vacuum arc remelting
Electroslag remelting (ESR))
used for smelting high-quality steels for bearings and heat-resistant steels. Metal melted in an arc furnace and rolled into a rod is subjected to remelting. The heat source is a slag bath heated by electric current. An electric current is supplied to the remelted electrode 1, immersed in a slag bath 2, and to a tray 9 installed in a slightly conical water-cooled crystallizer 7, which contains a seed 8. The released heat heats the bath 2 to a temperature above 1700 ºC and causes melting of the end of the electrode. Drops of liquid metal 3 pass through the slag and form a metal bath 4 under the slag layer. The transfer of metal drops through the main slag helps remove sulfur, non-metallic inclusions and gases from the metal. The metal bath is replenished by melting the electrode, and under the influence of the crystallizer it is gradually formed into an ingot 6. As the ingot is formed, either the tray is lowered or the electrode is raised. The oxygen content decreases by 1.5...2 times, sulfur by 2...3 times. The ingot is distinguished by density, uniformity, good surface quality, and high mechanical and performance properties. Ingots are produced in round, square and rectangular sections weighing up to 110 tons.
Vacuum arc remelting (VAR) is used to remove gases and non-metallic inclusions from metal.
The process is carried out in vacuum arc furnaces with a consumable electrode. The cathode is produced by mechanical processing of an ingot smelted in electric furnaces or ESR installations.
Consumable electrode 3 is fixed on a water-cooled rod 2 and placed in the furnace body 1 and then in a copper water-cooled mold 6. Air is pumped out of the furnace body to a residual pressure of 0.00133 kPa. When voltage is applied between the consumable electrode 3 (cathode) and the seed 8 (anode), an arc occurs. The generated heat melts the end of the electrode. Drops of liquid metal 4, passing through the arc discharge zone, are degassed, fill the mold and solidify, forming an ingot 7. The arc burns between the electrode and the liquid metal 5 in the upper part of the ingot throughout the entire melting. Cooling the ingot and heating the liquid metal creates conditions for directed solidification of the ingot. Consequently, non-metallic inclusions are concentrated in the upper part of the ingot, and the shrinkage cavity is small. The ingot is characterized by high uniformity of chemical composition and improved mechanical properties. It is used for the manufacture of parts for turbines, engines, and aircraft structures. The weight of the ingots reaches 50 tons.
Oxygen in steel
Before considering the results of studying the dynamics of oxygen content in steel during its production, the characteristics of secondary oxidation of the metal at various stages of the technological process, we present some judgments on these issues in the literature [1, 2].
The solubility of oxygen in solid iron and alloys based on it is very low; as a result, the metal at ordinary temperatures, as a rule, is supersaturated with oxygen. To prevent this phenomenon, steel deoxidation is carried out at the final stage of the steelmaking process. The content of oxygen dissolved in the liquid metal decreases and the chemical potential of oxygen in the metal ( M
O2 or
p02
equilibrium) becomes significantly lower than the chemical potential of oxygen in the phases in contact with the metal - the atmosphere, oxidizing slag, and in some cases even the refractory lining. As a result, the process of transition of oxygen from these phases into the bulk of the metal is established. The oxygen flow entering the metal is generally determined by the equation:
Processes of secondary oxidation of metal develop at the following stages of steel production processes: during the process of releasing metal from the unit into a ladle under the influence of atmospheric oxygen or slag iron oxides [3-5], with further oxidation of the metal by slag while holding the metal with slag in the ladle [6, 7], in the process of oxidation of the metal jet during casting or the metal surface in the casting device of a continuous caster or in a mold with air oxygen [8-12]. The share of each of these sources in the total effect of the secondary oxidation process can vary significantly depending on the technological features of the smelting and casting process. A characteristic feature of the secondary oxidation process is its practical uncontrollability, which causes increased and unstable waste of deoxidizers, contamination of the metal with non-metallic inclusions and, consequently, a decrease in the mechanical and other service properties of the finished metal.
Dynamics of changes in oxygen content during the smelting, processing and casting of steel.
It is known that the oxygen content, which largely determines the physicochemical properties of continuously cast steel, changes during the production process along the steelmaking unit-UPC-CCM route. We have studied the dynamics of changes in the oxygen content in the metal at the technological stages of its production. For this purpose, special samples were taken from the steel-smelting unit (before release), at the CPC - at the beginning, middle and end of processing, at the continuous caster from the tundish - at the beginning and end of casting. Samples were taken from melts of two groups of steel grades: 1-09G2S, S355, PCD 32; 2-A36, 3sp, S235.
The accumulated results of determining the oxygen content are presented in Figure 1.
Rice. 1
— Dynamics of oxygen content in the metal in the sections of the steelmaking unit - UPK - CCM
In the figure, the dotted line outlines the field within which the oxygen content fluctuates in various technological areas of steel production. Line 1 in the figure is the averaged data on the change in oxygen content in the steel-smelting unit, UPC and continuous caster for steel grade 09G2S, line 2 for 3sp, A36, S235.
From Figure 1 it can be seen that the oxygen content fluctuates within significant limits in almost all technological areas. The greatest scatter in oxygen content values and their highest value (220-420) ppm occurs during the period when the metal is in the steel-smelting unit before release.
At the beginning of processing (after deoxidation and alloying of Si and Mn and preliminary deoxidation of the Al metal when released into the ladle), the oxygen concentration decreases and the range of values narrows.
The lowest oxygen content values for both groups of heats were obtained at the end of metal processing at the UPC (20-50) ppm.
The beginning of steel casting at a continuous casting machine is accompanied by an increase in oxygen concentration and the spread of its values. This indicates the presence of secondary metal oxidation processes at this technological stage of production.