Steel has one drawback - it has magnetic properties, which are not always useful. Austenitic steel does not have this disadvantage. Such alloys have practically no magnetic properties, they do not rust, and withstand mechanical deformation well. Austenites are used for the production of radio equipment, turbines, and frost-resistant structures. What types of austenitic steels are there? How are various parts based on them welded?
General information
Austenitic steel is a special type of stainless steel. Austenitic steels contain iron, as well as various alloying components - nickel, manganese, nitrogen, aluminum, chromium, molybdenum.
Iron and alloying elements in steel form a cubic crystal lattice. This structure is called austenite. The crystal lattice determines a number of characteristic physical properties of austenite - preservation of hardness during heat treatment, almost complete absence of magnetic properties of the material, high chemical inertness.
For convenience, austenitic steels are divided into two conventional classes. The first category includes materials with a high nickel content. The second category includes materials with a high content of manganese and nitrogen, as well as a low nickel content.
The second materials have higher strength, but they cost an order of magnitude more. In addition, nickel-based austenite better withstands the effects of aggressive chemical environments (acids, alkalis, strong salts, radioactive substances).
Various techniques, things, and equipment are made from austenite steel. This could be metering devices, cutlery, metal beams, turbines, structural elements, automotive parts, special equipment for the needs of the chemical industry, and so on.
Another major application of austenite is the manufacture of radio equipment. The absence of magnetic properties in this case is beneficial - ordinary steel alloys can introduce certain distortions into the radio signal, while austenite will transmit the signal without delays, losses, or distortions.
Post-weld intergranular corrosion
Austenitic steels are often positioned as resistant to various types of corrosion. Intergranular corrosion, which occurs along the grains, often does not occur in the weld itself, but near the connection line and even at a considerable distance. In general, the physical aspects of the development of corrosion do not differ from each other - the only difference is in the cause of its occurrence.
Intercrystalline corrosion of the base metal occurs when a certain local area is simply overheated. For the weld material, from a physical and chemical point of view, everything is much more complicated. The thermal cycle of welding, as already mentioned, disrupts diffusion processes, as a result of which active carbon and alloying chromium are released onto the surface. They form one of those carbides that lead to increased brittleness of the weld. Obviously, in parallel with this, depletion of other alloying components occurs (carbon compounds with titanium and niobium are also formed to a lesser extent), and the material becomes more vulnerable to intergranular corrosion.
An obvious solution to reducing the susceptibility of the weld and heat-affected material to intergranular corrosion is austenitization at 1050-1100°C
An indirect solution to the problem is the introduction of austenitic-ferritic materials, which are more resistant not only to intergranular corrosion, but also to unfavorable thermal cycles. The special structure - up to 4% molybdenum and 25% chromium - is distinguished by smaller grains and, accordingly, an increased length of intercrystalline boundaries. An increase in the area of carbide precipitation leads to a decrease in their dispersity. Local depletion of chromium occurs to an insignificant depth. In addition, austenitic-ferritic materials have an increased rate of diffusion processes.
There are several ways to reduce the susceptibility of the weld and heat-affected material to intergranular corrosion. The obvious solution is to carry out austenitization, which is already known to us, at 1050-1100°C (can be replaced by stabilizing annealing for 2-3 hours at a temperature of 850-900°C). However, you can always come to terms with the precipitation of the carbide phase, neutralizing its consequences through additional alloying until an austenitic-ferritic structure is formed. The disadvantage of this approach is not only the excessive consumption of chromium and other metals, but also a decrease in the resistance to general corrosion that spreads over the entire surface of the product. In this light, some manufacturers prefer to use titanium, tantalum or vanadium as an alloying component instead of chromium. At the same time, more expensive metals also turn out to be more demanding in terms of the protective environment. If you do not use inert gases or fluoride fluxes instead of acidic ones, titanium, which is close to oxygen, burns out by 70-90%.
Additionally: it is necessary to debug automated processes that guarantee the continuity of obtaining a seam with a stable electric arc, the repeated excitation of which makes the thermal cycle unfavorable.
Physical properties
- High strength. Under normal operating conditions, the material retains its strength, elasticity, and stability. Therefore, the steel can withstand high loads. Strength is also maintained in the event of temperature changes - sudden cold snap, severe frosts, exposure to direct sunlight in summer, local slight heating and other situations.
- Magnetic inertia. The crystalline structure almost completely neutralizes the magnetic potential of iron and alloying elements. Therefore, when a magnetic element comes into contact with austenite, a very weak magnetic field is formed, which does not in any way affect the properties of the material.
- Corrosion resistance. Under normal temperature conditions, austenite steel does not come into contact with atmospheric oxygen, nitrogen, carbon dioxide, or water. Therefore, the risk of formation of destructive corrosive oxides is minimal. Austenitic steel can be used to make parts that will be used at offshore facilities (ships, bridges, turbines, metering devices).
- Chemical inertness. Steel, under normal temperature conditions, also does not react with various substances that have high chemical activity. Therefore, this material can be used for storage and work with acids, alkalis, salts, and radioactive substances. Chemical inertness is maintained even in case of prolonged contact. Therefore, austenite does not burst, does not rust, and retains its physical properties during prolonged contact with reagents.
Types of austenitic steels
Based on their composition and physical properties, there are 3 types of austenite steel:
Anti-corrosion austenitic grade steel
This category includes alloys with a high specific content of chromium and nickel. The alloy may also contain silicon, manganese, and molybdenum in small quantities. The peculiarity of alloys of this group is the minimal risk of corrosion at any temperature.
High stability is ensured due to two factors. The first factor is the high chromium content, which creates a protective film on the surface of the steel. The second factor is low carbon content (less than 0.3%). The combination of these factors leads to the fact that the material does not come into contact with oxygen, nitrogen, water, and various chemicals.
Stability is maintained even when heated or cooled, since chromium retains its physical properties when temperatures change.
Heat resistant class
This category includes alloys with a high content of nickel, boron, niobium, vanadium, molybdenum, and tungsten. Alloying components make the material more durable and minimize the risk of pores forming between individual iron atoms. Therefore, heat-resistant austenite retains its shape when heated to 1100 degrees.
Heat-resistant austenite material is suitable for the manufacture of various furnaces, machine tools, and factory equipment. Some alloys also contain large amounts of chromium. The result is a heat-resistant anti-corrosion alloy that not only withstands heat, but also does not corrode.
Cold resistant class
This category includes alloys with a high specific chromium content and an average nickel content. Aluminum, manganese, vanadium, and tungsten can be used as additional alloying additives.
Cold-resistant alloys can withstand very low temperatures and withstand sudden temperature changes. However, at normal room temperature, cold-resistant austenite steel has mediocre physical properties - low strength, weak chemical inertness.
Therefore, cold-resistant alloys are used to make special equipment for regions with very cold climates. Another area of application is the manufacture of parts, products, and equipment for the needs of the space industry.
Austenitic steels: composition and properties
Austenitic steel is a metal to which chromium and nickel have been added in percentages of 18% and 10% respectively. Because of this, they are also known by the digital abbreviation 18-10.
The main advantage of this class of steel is corrosion resistance due to the addition of chromium. The presence of a chromium additive in an amount of 18% makes the steel resistant to many oxidizing environments (for example, nitric acid).
Adding 9-12% nickel to steel transforms the material into the austenitic class. This process increases the usability of steel, namely increases ductility and reduces the likelihood of grain appearing.
Specific properties:
- heat resistance;
- heat resistance;
- cryogenicity;
- corrosion resistance.
Instead of chromium and nickel, austenitic steel may contain other additives: ferritizers and austenizers.
Welding austenitic steel
Welding technology can be used to connect austenite products. The joining of metals can be carried out by all main welding methods (electroslag, arc, gas-shielded).
Welding austenitic steels has many features and nuances that the welder needs to know about in advance. The peculiarity is a serious change in the physical properties of the austenite metal when heated. This imposes a number of requirements regarding welding. After all, if the metal is heated incorrectly, the quality of the weld seriously suffers, which will have a bad effect on the strength of the connection.
Features of austenite heating
- At a temperature of +350 degrees, active diffusion processes occur in the alloy, which leads not to an increase, but to a decrease in the ductility of the metal.
- From +350 to +500 degrees, thermal restructuring of the metal occurs. Such a physical process has a number of characteristic features - increased fragility of the material, cracking of carbide components, and changes in thermal conductivity.
- From +500 to +650 degrees, precipitation of carbide components occurs, which the welder must take into account during work.
- When the material is heated above +750 degrees, the fragility of the metal seriously increases. With such heating, small cracks can form on the metal, which reduces the strength of the weld.
However, the welder must avoid the appearance of cracks, irregularities, and holes in the weld area. To solve this problem, a small metal layer is fused onto the part in the weld area, which has a different chemical composition.
The patch layer requires a metal with increased heat resistance and high corrosion resistance. The patch will act as a protective layer that will prevent the seam from cracking. It is recommended to fire the protective layer at a temperature of +800 degrees to avoid the appearance of cracks under increased load levels.
Electroslag welding
Electroslag welding technology is suitable for joining both large and small austenite-based products. The main advantages of this technology are the minimal risk of cracks, the absence of deformation at the joints, and the ease of welding.
Welding is recommended to be carried out quickly and at low temperatures. Indeed, when the metal is heated for a long time above a temperature of 1200 degrees, local cracks can form, which can lead to the destruction of the metal.
A few additional notes regarding the use of electroslag technology:
- Welding is recommended to be done using wire, the thickness of which is 2-4 millimeters. The main disadvantage of this approach is that high-quality wire is consumed quickly, and it is quite expensive.
- To connect thick parts, plate electrodes should be used (optimal thickness - 5-15 millimeters). Electrodes are more expensive, but they degrade much more slowly.
- When working with alloys that have increased corrosion resistance, it is recommended to do hardening or annealing - this will help avoid the appearance of knife corrosion.
Arc welding
Arc welding for joining austenitic steel has many disadvantages.
Main disadvantage:
- During welding, the local region of the austenite metal is heated. Heat does two dangerous things that negatively affect strength.
- The first point is the appearance of iron oxides in the weld area. The physics of this process is as follows: when seriously heated, iron begins to come into contact with atmospheric air, which leads to the formation of oxides.
- The second point is the appearance of cracks near the seam. With high heating, the fragility of the material sharply increases while the overall ductility decreases, which contributes to the formation of small cracks.
Calcium fluoride electrodes
There are a number of techniques that can overcome the limitations of arc welding. The most popular method is the use of calcium fluoride electrodes of small diameter (the optimal cross-sectional diameter is 3-5 millimeters).
Such rods have low ductility, so during welding work the electrodes do not make unnecessary vibrations. This reduces the contact of the molten metal with air, and also reduces the risk of cracks due to increased brittleness.
1.5-2 hours before welding, it is recommended to calcinate calcium fluoride electrodes at a low temperature (200-300 degrees). This helps minimize the risk of porosity in the electrode.
Electric arc welding must be performed strictly with reverse polarity direct current. Otherwise, the stability of the electrode is not guaranteed.
Gas shielded welding
Welding austenitic steels using shielding gases is the best way to join austenites. This technique allows you to connect parts of various shapes, and welding can be carried out in any spatial position.
The use of shielding gases minimizes the likelihood of cracks, plaque, rust, and scale, which makes the welded joint very durable. Any gas can be used as a protective medium - argon, helium, nitrogen, carbon dioxide and others. For welding, consumable or tungsten rods are usually used, which are suitable for creating small, strong seams (the optimal thickness is 5-10 millimeters).
Features of austenite welding in shielding gases
- For welding work, you can use both a pulsed and a burning arc. However, experienced welders recommend using a pulsed arc. This reduces the thickness of the seam and minimizes the likelihood of edge crushing. Thanks to this, it is possible to obtain an even, strong seam that will not crack during long-term use of the product.
- It is recommended to weld austenite using direct current, which has straight polarity. If necessary, the polarity of the current can be changed - this will not affect the quality of the weld in any way. When choosing a burner, you need to pay attention to the type of polarity switching. After all, most burners work with devices that switch polarity automatically. If you want to change the polarity manually, you must read the instructions for the burner to make sure that it supports this mode of operation. Also note that when welding austenite with a high content of aluminum fillers, it is recommended to use an AC torch.
- For pulsed arc welding, it is recommended to use consumable electrodes. This connection method is suitable for connecting structures with a small thickness. These can be metal sheets, thin beams, and so on. The use of a consumable electrode minimizes the risk of cracks in the seam, which will have a beneficial effect on the shelf life of such a welded joint.
- Plasma welding of austenitic steels is permitted in situations where the thickness of individual welded elements is less than 15 millimeters. In the case of plasma welding of large objects, the risk of formation of undercuts and gaps increases sharply, which negatively affects the strength of the welded joint.
Study of the structure of welds in austenitic stainless steel
UDC 621.791:620.22
STUDYING THE STRUCTURE OF WELDS IN AUSTENITIC STAINLESS STEEL
©1
Irkutsk National Research Technical University,
Russian Federation, 664074, 3.
Annotation. The technological properties of austenitic steel 12Х18Н10Т are analyzed. The reasons for high corrosion resistance are indicated. The influence of alloying on mechanical properties and weldability is assessed. The microstructure of welded joints was studied.
Key words: pipeline, austenitic steel, microstructure, welded joint.
STUDY OF WELDED JOINTS STRUCTURE OF AUSTENITIC STAINLESS STEELS
N. Datsyuk
Irkutsk National Research Technical University,
83 Lermontov Str., Irkutsk, Russia, 664074
Abstract. The article analyzes the technological properties of austenitic steel 12x18h10t. The reasons of high corrosion resistance are specified in the article. The article assesses the impact of doping on mechanical properties and weldability. The article studies the microstructure of welded joints.
Keywords: pipeline; austenitic steel; microstructure; welded joint.
Introduction
Stainless steel 12Х18Н10Т occupies a leading position in the modern rolled metal market. Thanks to the exceptional combination of strength characteristics and properties, it is successfully used in almost all industries. Steel 12Х18Н10Т is used in the fuel and energy sector, in chemical engineering for the manufacture of containers designed to operate under high pressure, as well as in devices for the production of liquid oxygen. Along with other materials, this steel is widely used for the production of welded equipment and structures that, during operation, come into contact with corrosive environments [1–4]. It is known to use 12Х18Н10Т steel for elements of reaction, heat exchange and capacitive equipment, including high-pressure pipelines. Steam heaters are manufactured with a specified operating temperature of up to +600 °C, and in the presence of aggressive environments up to +350 °C. Parts for exhaust system manifolds, furnace equipment and muffles are made from steel of this grade. Chromium-nickel stainless steel is also used in cryogenic technology, the operating temperature of which reaches –169 °C. Semi-finished products are produced from it: sheets, circles, wire (including for welding), pipes. Meshes, springs, cables and ropes are made from steel threads. All products are characterized by a long service life. The objective of this study is to study the microstructure of welds made of steel 12Х18Н10Т.
Materials and research methods
In terms of structure, steel 12Х18Н10Т belongs to the austenitic class. The chemical composition is regulated by GOST 5632-72 “High-alloy steels and alloys, corrosion-resistant, heat-resistant and heat-resistant. Stamps." Table 1 shows the chemical composition of steel 12Х18Н10Т.
Table 1
Chemical composition of steel 12Х18Н10Т
| Mass fraction of elements, % | ||||||||
| C | Cr | Ni | Mn | S | P | Si | Cu | Ti |
| 0,12 | 17…19 | 9…11 | 0,2 | 0,02 | 0,035 | 0,8 | 0,3 | 0,4…1 |
Chromium, the content of which in steel is 17...19%, is the main element that ensures the metal's ability to passivate and ensures its high corrosion resistance.
Alloying with nickel in sufficient quantities (8...12%) transfers the steel to the austenitic class, which is very important: such steel combines high manufacturability with a unique set of performance characteristics. Austenitic steels have increased, compared to ferritic steels, corrosion resistance in a large number of aggressive environments, including sulfuric and a number of other acids. They roll well in hot and cold conditions, and are welded without embrittlement of heat-affected zones. The influence of nickel on corrosion resistance in steel of this class is manifested in the fact that, having increased resistance to acids, it imparts this property to steel.
In the presence of a small amount of carbon, the steel has a completely austenitic structure. The ratio of chromium and nickel concentrations has a specific effect on the stability of austenite when the treatment temperature is cooled to a solid solution (1050...1100 °C). Titanium eliminates the tendency to intergranular corrosion, since it is a strong carbide-forming element and during crystallization binds carbon into refractory carbide TiC, therefore eliminating the possibility of the formation of chromium carbides and a decrease in the chromium concentration in austenite.
Silicon degasses the metal and increases the density of the ingot. Because of silicon, the strength of steel increases, especially the yield strength increases, and there is a slight decrease in ductility, which makes cold rolling of steel more difficult. The introduction of manganese causes a slowdown in the rate of grain growth when heated, which leads to the production of fine grains.
Sulfur has unlimited solubility in liquid iron and limited solubility in solid iron. When steel crystallizes, iron sulfides, which are the last to solidify, are released along the grain boundaries. Iron and iron sulfides form a low-melting eutectic (Tm = 988 °C), which melts at even lower temperatures in the presence of oxygen. Between the grains of the alloy, layers of a sulfur-enriched phase are formed, which, when the metal is heated before rolling or forging, soften and the steel loses its properties and red brittleness occurs. The sulfur content in steel 12Х18Н10Т is limited to 0.02%.
Phosphorus has a negative effect on the mechanical properties of steel, since strong primary segregation occurs during crystallization. The brittle layers rich in phosphorus located between the grains reduce the plastic properties of the metal, especially at low temperatures (cold brittleness). The permissible phosphorus content in steel 12Х18Н10Т is not more than 0.035%. In this case, this is critical, because steel 12Х18Н10Т is used in cryogenic technology.
During crystallization, in the absence of elements that form nitrides at high temperatures (this steel contains Ti), after the formation of r-Fe, nitrogen begins to be released from the solution in the form of inclusions (iron nitrides). This release can continue for a considerable time, causing embrittlement of the metal (aging). The deterioration of the properties of metal, which contains a lot of nitrogen, is especially harmful when used at low temperatures.
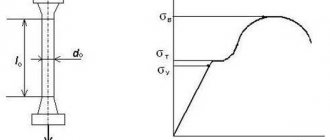
Table 2 shows the mechanical properties of steel 12Х18Н10Т after hardening at a temperature of 1050...1100 °C and subsequent cooling in air.
table 2
Mechanical properties of steel 12Х18Н10Т
| Test temperature, °C | Conditional yield strength, , MPa | Tensile strength, MPa | Relative elongation, d, % | Relative narrowing, Ш, % | Impact strength, KCU, kJ/cm2 |
| 20 | 225…315 | 550…650 | 46…74 | 66…80 | 215…372 |
| 500 | 135…205 | 390…440 | 30…42 | 60…70 | 196…353 |
| 700 | 120…195 | 265…360 | 20…38 | 40…70 | 255…353 |
As can be seen from the table, steel at high temperatures retains sufficient strength and high toughness. After heat treatment, consisting of quenching from 1050 °C followed by cooling in water, the steel has a homogeneous structure of a r-solid solution. In steel, when cooled to –196 °C, no transformations occur, just as when heated to hot plastic deformation. But with long-term holding of hardened steel in the range of 450...650 °C, the formation of chromium carbides of the Cr23C6 type is observed in the structure, which causes a tendency to intergranular corrosion with a minimum incubation period at 600 °C equal to 8...10 hours (tests were carried out in boiling 65% - nitric acid, three cycles of 48 hours).
Another disadvantage of stainless steel is the difficulty of machining it. Stainless steels, like many aircraft materials, are difficult to cut. Exposure to high cutting forces and temperatures causes accelerated wear of the cutting edges of metalworking tools. To increase the durability of tools used for processing stainless steels, strict adherence to hardening heat treatment regimes, the creation of a tool with a special cutting edge geometry, and compliance with cutting conditions are required [5–9].
Steel 12Х18Н10Т welds well, without any restrictions [10–12]. When welding steel 12Х18Н10Т, it is recommended to use electrodes with a basic type of coating in combination with a high-alloy electrode rod [11]. Argon is most often used as shielding gases for arc welding, and helium and carbon dioxide are less commonly used.
experimental part
Manual arc welding was used to produce welds using direct current of reverse polarity using TsL-11 electrodes with a basic coating. Before welding, the electrodes were calcined for 1.5 hours at a temperature of 190...210°C.
Welding in argon was carried out using a Kemppi Kempomat 3200 semi-automatic welding machine. The wire used was Sv-06Х19Н9Т. Microsections were made from welds, and their microstructure was studied [2, 3, 13, 14]. The sample was cut out using an abrasive wheel and filled with epoxy resin. After hardening, grinding and polishing were carried out. Etching was carried out with an aqueous solution of a mixture of hydrofluoric and nitric acids. The microstructure was studied using a metallographic microscope at different magnifications. Figure 1 shows the microstructure of welds.
| A | b |
| Fig.1. Microstructure of a welded seam in steel 12Х18Н10Т: a – RDS, x100; b – welding in argon, x200 |
When welding in argon, a homogeneous weld structure is obtained with a gradual transition of the structure from the base metal to the weld metal.
With RDS, a layered structure is observed in the weld structure. The samples contain a larger number of pores. In the weld metal, dendrites of a solid solution of alloying elements in iron—austenite—are observed.
Conclusion
Welding in argon ensures the highest quality welded joints. While performing this work, a technique for preparing welds for microstructural studies was mastered.
Bibliography
Stainless steels. M.: Metallurgy, 1967. 799 p. , , Metallographic study of welded joints made of steel 12Х18Н10Т, made by various types of welding // In the collection: Life cycle of structural materials (from receipt to disposal) materials of the III All-Russian scientific and technical conference with international participation. Irkutsk: ISTU Publishing House, 2013. pp. 133–140. , Assessment of residual stresses in surfacing of high-pressure valve seats // Chemical and oil and gas engineering. 2022. No. 7. pp. 26–29. , Analysis of modification of a high pressure valve using Rollscan 300 // Youth Bulletin of ISTU. 2016. No. 3. P. 8. , Application of innovative means for quality control of tools made of high-speed steels // Modern technologies. System analysis. Modeling. 2016. No. 2 (50). pp. 73–80. , , Production of high-performance cutting tools in IAZ conditions // Science and technology in industry. 2013. No. 1,2. pp. 91–95. , Application of magnetic methods for quality control of products made of tool steels // In the collection: Life cycle of structural materials (from receipt to disposal) materials of reports of the II All-Russian scientific and technical conference with international participation. 2012. pp. 338–344. Nikolaev A. Yu. Simulation of the plain milling process // In the collection: IOP Conference Series: Materials Science and Engineering 10. Ser. “International Conference on Mechanical Engineering, Automation and Control Systems 2016” 2022. P. 012080. Nikolaeva EP, Vlasov DB Effect of heat treatment conditions on structure and properties of high-speed steel // In the collection: IOP Conference Series: Materials Science and Engineering 10. Ser. "International Conference on Mechanical Engineering, Automation and Control Systems 2016" 2022. P. 012113. Welding of high-alloy steels. Kyiv: Technique. 1975. 376 p. Welding in mechanical engineering: Handbook in 4 volumes / Vol.2. Ed. . M.: Mechanical Engineering, 1978. 462 p. Welding of heat-resistant austenitic steels and alloys. M.: Mechanical Engineering, 1966. 429 p. Study of the structure of structural steel St3ps after treatment with argon arc plasma // In the collection: Prom-Engineering proceedings of the II international scientific and technical conference. FSBEI HPE "South Ural State University" (national research university). 2016. pp. 191–195. Nikolaeva EP Structure investigation of the constructional steel St3ps after argon-arc plasma treatment // Materials Science Forum. 2016. T. 870. pp. 500–506.
1, master's student at INRTU, *****@***ru
Datsyuk Nikita, Undergraduate of IRNITU, *****@***ru
GOST standards
The production of austenite is regulated by legislative norms, rules, and laws. The main standards are listed in the following regulatory documents - GOST 5632-2014, GOST 11878-66, GOST R ISO 4136-2009.
These documents define all the main points that relate to austenitic steels - production, marking, categories, grades, transportation features, and so on.
In accordance with GOST standards, metallography or magnetic technology can be used to determine the content of ferritic (iron) components in any austenite-based products. To carry out the test, small rods (at least 2 pieces) are cut from austenite.
Check algorithm
- Determination of iron content by metallography. Small sections are made on the rods, which are subjected to electrolysis or chemical etching. After this, the thin sections are placed under a powerful microscope, where the content of ferrous compounds is visually determined. Based on the research results, a rating is given that determines the concentration of iron in the base alloy. To increase the accuracy of the research, it is recommended to take several independent samples from several rods.
- Determination of iron content by magnetic method. Micro-sections are made on the rods, which are ground and cleaned using abrasive materials. After this, a series of measurements is carried out using ferritometers with a high sensitivity threshold. The minimum number of measurements is 40 pieces. At the end, the information obtained is processed using methods of mathematical statistics and modeling. To increase the accuracy of the study, it is recommended to take several independent samples.