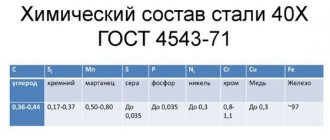
Main consumers of quenching oils
OJSC "AVTOVAZ" CJSC "Chelyabprommash" Kama Automobile Plant - KAMAZ Likhachev Plant - ZiL Cheboksary Tractor Plant - ChZPT Chelyabinsk Tractor Plant - ChTZ Vladimir Tractor Plant - VTZ Volgograd Tractor Plant - VgTZ Lipetsk Tractor Plant - LTZ Rostselmash Ural Heavy Engineering Plant Izh Orsky plants Novokramatorsky machine-building plant Krasnoyarsk Heavy Engineering Plant OJSC "PO SEVMASH" OJSC "East-Siberian Machine-Building Plant"
What is metal hardening oil?
To obtain metal of maximum hardness, strength and wear resistance, a process of heating to a certain temperature occurs, then cooling in a liquid. The cooling material for hardening metal is quenching oils. Thanks to the use of quenching oil, it is possible to produce steel products with a given plane accuracy, required strength and a certain structure.
Quenching oils – evrosmazki.ua
How to drill through hardened metal
First of all, we list the main features of drilling workpieces and products made of hardened metal. For successful processing you need:
- choose the right drill;
- prepare a workpiece or product;
- use cutting fluid.
Which tool to choose for drilling hardened metal
For drilling hardened metal, tools made from the following grades of steel are best suited.
- P18. Tools made from steel of this brand are the best choice. These drills for hardened metal appeared back in Soviet times. The material contains up to 18% tungsten. This gives the steel high strength. The surfaces do not overheat and wear out slowly.
- R6M5K5. This grade of steel contains 6% tungsten and 5% each of molybdenum and cobalt. These hardened metal drills can withstand maximum heat loads when machining hardened parts and products.
- HSS-Co. This is a foreign analogue of the previous steel.
Hardened metal drill made from HSS-Co steel
Craftsmen choose drills made from these steel grades because of the optimal combination of price and efficiency in processing high-strength hardened metals.
Note! Before drilling, it is necessary to thoroughly clean the workpiece or product from oils, grease and other contaminants.
Tips for using coolant when processing hardened metal
- Add coolant to tool cutting edges. During processing, the liquid scatters and evaporates. The lubricant must be updated promptly.
- Before processing a part or product, it is also necessary to apply coolant to the target surface.
- When drilling hot metal, take short breaks to allow the workpiece and tool to cool.
Physical properties of cooling oils
Quenching oils must meet the requirements of GOST in terms of physical and chemical parameters. The liquid is characterized by a long cooling time for the metal. The oil must have the following physical properties:
- high resistance to evaporation;
- absence of impurities, water and other chemicals;
- high chemical resistance (maintaining properties throughout the entire period of use);
- have excellent cleaning properties;
- high ignition temperature when heated.
have a certain level of viscosity;
Types of quenching oils
The domestic chemical industry began producing high-quality oils for metal hardening at the end of the last century. Additives are added to the quenching oil to increase oxidation resistance, reduce foaming, and have excellent cleaning properties.
Industrial oils have different applications and are accordingly used in the processing of metals with high physical requirements:
- For grinding high-alloy, high-strength steel grades, oil is used to increase the cold hardening rate.
- To create small mechanical parts from low-strength steels, low volatility oil is used.
- Hardening of volumetric parts made of alloy steels occurs using oil with excellent oxidative stability and viscosity, maximum ignition temperature.
Quenching oils – evrosmazki.ua
Types of Heat Treatment
Heat treatment, which is what this process is also called, comes in several types: processing with and without polymorphic circulation. The first type of heat treatment is used primarily for some alloys and non-ferrous metals, and the second is used for steel products.
During heat treatment, a metal product acquires improved hardness, and its initial ductility is significantly reduced. The viscosity of the material also decreases. The decrease will be even greater if the cycle is repeated repeatedly. The process of so-called “tempering” allows you to reduce brittleness and slightly improve viscosity. It affects the reduction of acquired strength, but the reduction rates will be insignificant. When tempering metals without polymorphic treatment (alloys and non-ferrous metals), a process called “aging” is used.
Introduction
There is a characteristic of steel - hereditary and acquired grain size. The grain size can be smaller or larger, and it also changes under the influence of high temperatures. How quickly depends on the amount of impurities. It is impossible to say unambiguously which crystal lattice and which compounds are better. In some cases, strength depends on this, in others ductility. This indicator must be changed depending on what kind of processing is to be done. If sheet steel or a profile is planned to be cut, then a procedure should be carried out that leads to grain coarsening. And if the work is to be done with high-carbon steel, then workpieces with a fine-grained structure are better processed.
Changing the grain size is quite difficult. In this case, hereditary propensity must be taken into account. This does not mean that the alloy will in any case have large grains, but with the same heating of two bars with different heredity, one will produce growth of compounds faster than the other. Therefore, the factor is very important when selecting heating. So not everyone can only selectively harden metal at home; you should know the chemical composition.
The alloy has many impurities. Among them:
- Ferrite. This is the fundamental element that is most abundant. It carries the basic properties, other substances only increase or decrease them.
- Perlite. Increases hardness and tensile and compressive strength.
- Cementite. Chemical formula – iron with carbon. And although element “C” increases the strength characteristics, if you use pure FeC, you may be surprised at its fragility.
- Graphite. High-carbon Damascus steels are obtained by saturation with this impurity at the time of processing by forging.
- Austenite. Formed at a moment of very high heat. At the same time, plasticity increases, and magnetic properties disappear.
If the carbon content is from 0% to 2.18%, then we are dealing with steel - low carbon (up to 0.8%) or carbon. And if it is more than 2.18%, then we have durable cast iron. We conclude: the characteristics depend on two reasons:
- amount of impurities;
- degree of thermal treatment.
And if you can’t change the first one yourself, then the second one certainly can.
Types of hardening
Hardening can be either complete or incomplete. With complete hardening, significant changes occur in the structure of the material and the crystal lattice. Partial hardening is often applied to various types of tool steel products.
There are also products that require only partial hardening. That is, when it is necessary to harden not the entire metal, but a small part, for example, a cutting edge (this is how swords, various cutters for machine tools, drills and much more are hardened). On such a metal the boundary is clearly visible.
Microstructure of steel after hardening
Most steels after hardening are characterized by the structure of martensite and retained austenite, the amount of the latter depending on the carbon content and the qualitative and quantitative content of alloying elements. For structural steels of medium alloying, the amount of retained austenite can be in the range of 3-5%. In tool steels this amount can reach 20-30%.
In general, the structure of steel after hardening is determined by the final requirements for the mechanical properties of the product. Along with martensite, after quenching, ferrite or cementite may be present in the structure (in case of incomplete quenching). When steel is isothermally hardened, its structure may consist of bainite. The structure, final properties and hardening methods of steel are discussed below.
Partial hardening of steel
Partial quenching is called quenching, in which the cooling rate is not sufficient for the formation of martensite and it turns out to be below critical. This cooling rate is indicated by the blue line in the figure. During partial hardening, the “nose” of the C-curve steel seems to be touched. In this case, in the structure of the steel, along with martensite, troostite will be present in the form of black island inclusions.
The microstructure of partially hardened steel looks something like this:
Partial hardening is a defect that is eliminated by complete recrystallization of the steel, for example, during normalization or during reheating for hardening.
Incomplete hardening of steels
Quenching at temperatures lying between A1 and A3 (incomplete quenching) retains in the structure of hypoeutectoid steels, along with martensite, part of the ferrite, which reduces the hardness in the quenched state and worsens the mechanical properties after tempering. This is understandable, since the hardness of ferrite is 80HRC, and the hardness of martensite depends on the carbon content and can be more than 60HRC. Therefore, these steels are usually heated to temperatures 30–50 °C above A3 (full hardening). In theory, incomplete hardening of steels is not permissible and is considered a defect. In practice, in some cases, incomplete quenching can be used to avoid quenching cracks. Very often this concerns hardening with high frequency currents. With such hardening, it is necessary to take into account its feasibility: type of production, annual program, type of product responsibility, economic justification. For hypereutectoid steels, quenching at temperatures above A1 but below Acm produces excess cementite in the structure, which increases the hardness and wear resistance of the steel. Heating above the temperature Acm leads to a decrease in hardness due to the dissolution of excess cementite and an increase in retained austenite. In this case, the austenite grain grows, which also negatively affects the mechanical characteristics of the steel.
Thus, the optimal quenching for hypoeutectoid steels is quenching from a temperature 30–50 °C above A3, and for hypereutectoid steels – at 30–50 °C above A1.
The cooling rate also affects the hardening result. The optimal cooling medium is one that quickly cools the part in the temperature range of minimum stability of supercooled austenite (in the range of the nose of the c-curve) and slowly in the temperature range of martensitic transformation.
This is interesting: Designation and image of the thread in the drawing according to GOST
Cooling stages during hardening
The most common quenching media are water of various temperatures, polymer solutions, alcohol solutions, oil, molten salts. When hardening in these environments, several cooling stages are distinguished:
- film cooling, when a “steam jacket” forms on the surface of the steel;
- nucleate boiling, which occurs with the complete destruction of this steam jacket;
- convective heat transfer.
In addition to liquid quenching media, cooling in a gas flow of different pressures is used. It can be nitrogen (N2), helium (He) and even air. Such quenching media are often used in vacuum heat treatment. Here it is necessary to take into account the fact of the possibility of obtaining a martensitic structure - the hardenability of steel in a certain environment, i.e. the chemical composition of the steel on which the position of the c-curve depends.
Hardening technology
Thermal hardening technology involves rapid cooling that can reduce the temperature of the product to 600 – 410°C. The time required for sufficient heating of a metal product directly depends on the heating device used. When heating the material in a powerful electric furnace, it takes 1–2 minutes to heat treat 1 mm. With the same process in an oven with an open flame, one minute is enough, and when heated in a bath with a salt solution, about half a minute. The minimum time required for hardening is using a bath with a lead solution, in which 6 seconds is enough.
When hot metal is immersed in a quenching liquid, film vapor is formed, which leads to a significant reduction in the cooling rate. The temperature in the film vapor increases significantly, and when it breaks, the liquid begins to boil on the surface of the hot metal and cools it sharply. This process is called bubble boiling.
When the metal cools, the liquid stops boiling, and therefore the cooling process itself slows down significantly. At this time, a process of convective heat exchange occurs in the metal product.
Hardening methods
The essence of any hardening is the transformation of austenite into martensite (iron-carbon diagram). Depending on the temperature regime, hardening can be complete or incomplete. The first method is to harden tool steel, and the second is to harden non-ferrous steel.
During hardening, one or more coolants can be used. The method of heat treatment also depends on this. Depending on the cooling medium, heat treatment of the metal can be:
- using one cooler;
- with cooling;
- intermittent;
- stepped;
- isothermal.
Quenching in one cooler
This method is used for heat treatment of simple parts made of alloy and carbon steel. The part is heated to the required temperature and then cooled in liquid. Carbon steel with a diameter of 2 to 5 mm is cooled in water, parts of smaller diameter and all alloy steel are cooled in oil.
Hardening with cooling
When heat treating with a single coolant, thermal and structural internal stress conditions often occur. They develop when the temperature difference reaches a minimum. Tensile stress is formed on the surface of the metal, and compressive stress is formed in the center. To reduce these stresses, before lowering the heated part into the liquid, it is kept in the open air for a short time. The temperature of the part in this case should not be below the 0.8 K line on the iron-carbon diagram.
Intermittent
This hardening is carried out in two environments - water and oil or water and air. A part heated to a critical point is first quickly cooled in water, and then slowly in oil or in the open air. This heat treatment method is used for high-carbon steel. This method is complex, since the cooling time in the first environment is very short and only a highly qualified specialist can determine it.
Stepped
With intermittent heat treatment, the part cools unevenly—thinner surfaces cool faster than others. In addition, it is very difficult to adjust the time the part is in the first medium (water). Therefore, it is better to use step hardening. This method allows the part to be cooled in an environment at a temperature above the martensitic point. The first stage is cooling and holding the part in a given environment until all sections of the part reach the same temperature. The second stage is the final slow cooling (transformation of austenite into martensite).
Isothermal
In isothermal heat treatment, the part is heated to a critical point and then lowered into an oil or salt bath at a temperature of 250 degrees. Leave for half an hour and then cool in the open air. This hardening provides high structural strength and is used for alloy and structural steels in which the decomposition of austenite in the intermediate region does not completely occur. Subsequently, it turns not into martensite, but into bainite + 20% retained austenite, enriched in carbon. With this hardening, high strength and good toughness can be achieved.
How to take a holiday on your own
Tempering of steel is carried out to reduce its brittleness and increase its ductility, which occurs during its heating to a low (compared to quenching) temperature, followed by slow cooling.
Most steels (carbon and low alloy) that can be hardened in a home workshop are tempered at temperatures ranging from 150 to 250 °C (see table above). Unlike hardening, such heating does not require special equipment, so many home craftsmen use household stove ovens with thermostats for this purpose.
The heating temperature during tempering can be determined by the color of the tarnish - a multi-colored oxide film that appears on the surface of the steel when heated (see figure below). If you harden steel “to martensite,” that is, with rapid cooling in water, you will get a very hard but brittle structure. Therefore, tempering is a mandatory procedure when heat treating a cutting tool.
VIEW Melting furnace on AliExpress →
Temperature
Hardening is the transformation of austenite into martensite. In production, when choosing heat treatment temperatures, the iron-carbon diagram is used. The hardening temperature of carbon steels is very easy to determine. Heating of structural steel with a carbon content of less than 0.8% is brought to temperatures located above the GS line and above the Ac3 point by 30-50 degrees. Heating of steels containing more than 0.8% carbon is carried out at temperatures 30-50 degrees higher than those located above the PSK line. The hardening temperature of alloy steel is also selected based on the critical points, but this process is much more complicated, since in addition to carbon, such steels also contain other components.
Martensite and martensitic transformation in steels
Martensite is a supersaturated solid solution of carbon in α-iron (α-Fe). Read what austenite, cementite, ferrite and pearlite are here. When eutectoid steel (0.8% carbon) is heated above point A1, the original pearlite structure will transform into austenite. In this case, all the carbon present in the steel will dissolve in austenite, i.e. 0.8%. Rapid cooling at a supercritical rate (see figure below), for example in water (600 °C/sec), prevents the diffusion of carbon from austenite, but the fcc crystal lattice of austenite will rearrange into the tetragonal lattice of martensite. This process is called martensitic transformation. It is characterized by the shear nature of the restructuring of the crystal lattice at a cooling rate at which diffusion processes become impossible. The product of martensitic transformation is martensite with a distorted tetragonal lattice. The degree of tetragonality depends on the carbon content in the steel: the more it is, the greater the degree of tetragonality. Martensite is a hard and brittle structure of steel. Found in the form of plates, under a microscope they look like needles.
The hardening temperature for most steels is determined by the position of the critical points A1 and A3. In practice, the hardening temperature of steels is determined using steel graders. How to choose the hardening temperature of steel, taking into account points Ac1 and Ac3, read the link.
Coolant selection
The quality of the part depends on the choice:
- clean water is used to cool simple parts and products made from carbon steel;
- For products of complex shapes, caustic soda mixed with water in a 1:1 ratio is used as a coolant. The prepared solution is heated to 50-60 degrees;
- Hardening of metal in oil is applicable to thin-walled parts made of alloy or carbon steels.
Carbon steel, which has a complex composition, is cooled in two coolers - first quickly in clean water, and then slowly in a bath filled with oil. You need to move parts from water to oil very quickly.
Common media for self-heating
For hardening steel at home, the following cooling media are usually used: air, water and aqueous solutions, mineral oil. 10-15% sodium chloride (table salt) is usually used as aqueous solutions, and mineral oil in home workshops is most often used as a regular engine oil. To harden individual parts of a product with different hardness, hardening with sequential cooling in two environments is used. Each of these quenching media is characterized by its own cooling rate, which directly affects the structure of the metal being processed. For example, air cools steel at a rate of 5÷10 °C per second, oil - 140÷150 °C, and water (depending on temperature) - 700÷1400 °C.
In order to properly and without problems harden your product, you need to know the brand of metal from which it is made, since both the heating temperature and the cooling method depend on this. For their products, craftsmen most often use used products made from high-speed and tool steels, which can be hardened in a home workshop, as starting materials. The table below shows the recommended temperature conditions and cooling environments for various steels.
Hardening metal in oil
Oil conducts heat rather poorly, which contributes to the slower formation of structural elements of steel. Therefore, if it is hardened in an oil environment, it will acquire strength and elasticity along with hardness. In production, industrial oil I-20 or modern quenching oils such as “Thermoyl”, “Thermo” or “Voltex” are usually used for hardening. In home workshops, craftsmen use what is available. Most often this is new or used motor oil. To safely quench a part in such oil at home, you need to remember that it has a much lower flash point compared to industrial quenching liquids, and when hot metal is immersed in it, it ignites for a short time, releasing acrid smoke. Therefore, a hardening container used in a home workshop should have a minimal exposed surface and be used only outdoors or in a ventilated area. In addition to ordinary buckets and cans, one of the most common designs of such containers, which are used by home craftsmen, is an elongated section of pipe of a suitable diameter with a welded bottom.
This is interesting: Brass - the composition of the alloy, or the advantages and disadvantages of the metal
What are the defects when hardening metal?
If the hardening conditions are not followed, the following defects may appear:
- cracks or warping. The reason is internal tension. If the warping can be straightened and straightened, then the cracks cannot be corrected in any way. This is the final marriage;
- burnout, which is oxides along the grain boundaries, resulting from the penetration of oxygen into metal products. Burnout is possible when the metal is heated to a temperature close to the melting point. Such metal cannot be repaired;
- overheat. When a metal is heated above the incandescent temperature, it overheats, resulting in the formation of a large structure. This metal is highly brittle. Corrected by annealing and new hardening;
- low hardness. Insufficient hardness is obtained at low heating temperatures, insufficient holding at the required temperature and low cooling rates. This defect can be easily corrected by annealing and further hardening;
- oxidation and decarbonization, which occurs when the metal is exposed to air and furnace gases. The oxidized layer, scale, causes irreparable harm to production, because such a defect cannot be corrected. To avoid this problem, it is necessary to use ovens with a protective atmosphere.
How to harden metal yourself at home
Heat treatment of metals is one of the main ways to improve their mechanical and physical-chemical characteristics: hardness, strength and others.
One type of heat treatment is hardening. It has been successfully used by man in a handicraft way since ancient times. In the Middle Ages, this method of heat treatment was used to improve the strength and hardness of metal household items: axes, sickles, saws, knives, as well as military weapons in the form of spears, sabers and others.
And now they use this method of improving the characteristics of metal, not only on an industrial scale, but also at home, mainly for hardening metal household items.
What is metal hardening and its types
Hardening is understood as a type of heat treatment of a metal, consisting of heating it to a temperature, upon reaching which a change in the structure of the crystal lattice occurs (polymorphic transformation) and further accelerated cooling in water or an oil medium. The purpose of this heat treatment is to increase the hardness of the metal.
Hardening is also used, in which the heating temperature of the metal prevents a polymorphic transformation from taking place. In this case, its state is recorded, which is characteristic of the metal at the heating temperature. This state is called a supersaturated solid solution.
Polymorphic transformation hardening technology is used mainly for products made of steel alloys. Non-ferrous metals are subjected to hardening without achieving a polymorphic change.
After such treatment, steel alloys become harder, but at the same time they become more brittle, losing their ductility.
To reduce unwanted brittleness after heating with polymorphic change, a heat treatment called tempering is used. It is carried out at a lower temperature with gradual further cooling of the metal. In this way, the stress of the metal is relieved after the hardening process, and its fragility is reduced.
When hardening without polymorphic transformation, there is no problem with excessive brittleness, but the hardness of the alloy does not reach the required value, therefore, during repeated heat treatment, called aging, it is, on the contrary, increased due to the decomposition of the supersaturated solid solution.
Features of steel hardening
Mainly stainless steel products and alloys intended for their manufacture are hardened. They have a martensitic structure and are characterized by increased hardness, leading to brittleness of products.
If you heat treat such products by heating to a certain temperature followed by rapid tempering, you can achieve an increase in viscosity. This will allow the use of such products in various fields.
Types of steel hardening
Depending on the purpose of stainless steel products, it is possible to harden the entire item or only that part of it that must be functional and have increased strength characteristics.
Therefore, hardening of stainless steel products is divided into two methods: global and local.
:
Cooling medium
Achieving the required properties of stainless materials largely depends on the choice of cooling method.
Different grades of stainless steel undergo cooling differently. If low-alloy steels are cooled in water or its solutions, then for stainless alloys oil solutions are used for these purposes.
Important: When choosing a medium in which to cool the metal after heating, it should be taken into account that cooling occurs faster in water than in oil! For example, water at a temperature of 18°C can cool an alloy by 600°C in a second, but oil by only 150°C.
In order to obtain high metal hardness, cooling is carried out in running cold water. Also, to increase the hardening effect, a brine solution is prepared for cooling by adding about 10% table salt to the water, or an acidic medium containing at least 10% acid (usually sulfuric) is used.
In addition to the choice of cooling medium, the cooling mode and speed are also important. The temperature decrease rate must be at least 150°C per second.
Thus, in 3 seconds the temperature of the alloy should drop to 300°C. Further reduction in temperature can be carried out at any speed, because
The structure fixed as a result of rapid cooling at low temperatures will no longer be destroyed.
Important: Cooling the metal too quickly leads to its excessive fragility! This should be taken into account when hardening yourself.
The following cooling methods are distinguished:
- Using one medium, when the product is placed in a liquid and kept there until completely cooled.
- Cooling in two liquid media: oil and water (or saline solution) for stainless steels. Products made of carbon steel are first cooled in water, since it is a fast cooling medium, and then in oil.
- Using the jet method, when the part is cooled with a stream of water. This is very convenient when you need to harden a specific area of the product.
- Using the method of step cooling in compliance with temperature conditions.
Temperature
The correct temperature regime for hardening stainless steel products is an important condition for their quality. To achieve good characteristics, they are uniformly heated to 750-850°C, and then quickly cooled to a temperature of 400-450°C.
Important: Heating the metal above the recrystallization point leads to a coarse-grained structure, which worsens its properties: excessive brittleness, leading to cracking!
To relieve stress after heating the metal to the desired hardening temperature, step-by-step cooling of products is sometimes used, gradually lowering the temperature at each heating stage. This technology allows you to completely remove internal stress and obtain a durable product with the required hardness.
How to harden metal at home
Using basic knowledge, you can harden steel at home. Heating of metal is usually carried out using a fire, electric muffle furnaces or gas burners.
Hardening an ax at the stake and in the oven
If you need to give additional strength to household tools, for example, to make an ax more durable, then the easiest way to harden it can be done at home.
During manufacture, axes are stamped with a mark by which you can identify the grade of steel. We will look at the hardening process using U7 tool steel as an example.
The technology must be performed in compliance with the following rules:
1. Annealing . Before processing, dull the sharp edge of the blade and place the ax in a burning brick oven to heat. The heat treatment procedure must be carefully monitored to prevent overheating (permissible heating is 720-780°C). More advanced craftsmen recognize the temperature by the color of the heat.
And beginners can find out the temperature using a magnet. If the magnet stops sticking to the metal, it means the ax has heated up above 768°C (red-burgundy color) and it’s time to cool down.
Use a poker to move the hot ax to the oven door, remove the heat deeper, close the door and valve, leave the heated metal in the oven for 10 hours. Let the ax gradually cool down with the stove.
2. Hardening of steel . Heat the ax over a fire, potbelly stove or stove until dark red - temperature 800-830°C (the magnet has stopped magnetizing, wait another 2-3 minutes).
Quenching is performed in heated water (30°C) and oil. Lower the ax blade into the water 3-4 cm, moving it vigorously.
Next, place the ax in a container with oil; if the oil catches fire, cover the container with a thick cloth. Keep in oil until completely cool.
3. Release of the ax blade . Tempering reduces the brittleness of steel and relieves internal stress. Sand the metal with sandpaper to better distinguish the colors of the paint.
Place the ax in the oven for 1 hour at a temperature of 270-320°C. After standing, remove and cool in air.
: heat treatment of an ax at home, three stages: annealing, hardening, tempering.
Hardening the knife
It is advisable to use furnaces to harden metals yourself. For household items in the form of knives, axes, drills and others, small muffle furnaces are the most suitable. In them you can achieve a hardening temperature much higher than on a fire and it is easier to achieve uniform heating of the metal.
You can make such a stove yourself. You can find many simple design options on the Internet. In such ovens, a metal product can be heated to 700-900°C.
Let's look at how to harden a stainless steel knife at home using an electric muffle furnace. For cooling, instead of water or oil, melted sealing wax is used (can be obtained from a military unit).
The sequence of the hardening process is as follows:
- the knife (without a handle, if it is wooden) is placed in a cold oven;
- turning on the closed oven, heat it together with the knife until the blade turns bright red (800-900°C);
- Using a hot knife blade, cut the sealing wax up to 10 times, plunging 1.5 cm into it;
- the procedure is repeated up to 5 times, heating the knife blade and cooling in wax;
- Remains of sealing wax are removed with turpentine using a dampened cloth.
It is better to do the procedure in the fresh air; sealing wax smells terrible when melted. Also, the knife blade can be heated over an open fire.
: other ways to harden a knife at home.
PS Knowing the behavior of metal when heated and its properties after heat treatment, as well as the technology of hardening, you can successfully carry it out at home to improve the characteristics of small-sized metal products.
(3 5,00 of 5) Loading...
Hardening steel at home or cottage
Sometimes it happens that heat treatment of metal at home or in the country is necessary. This happens if the purchased tool turns out to be under-hardened or not hardened at all. Often there is a need to harden a knife, ax or drill. Of course, good hardening can only be done under production conditions, but male craftsmen are excellent at doing this over an ordinary fire. Sequence of home hardening:
- prepare two containers. Pour mineral oil into one, water into the other;
- We also need to prepare a tool with which we will put the metal to be hardened into the fire and remove it from it. Pincers are suitable for this procedure;
- Next, we make a fire and wait for the coals to form. We place a metal object on them that needs to be hardened;
- We monitor the color of the coals and the color of the flame. Hot coals are white. And the flame should not be white. The crimson color of the flame is optimal for the hardening process at home. A white flame indicates that the temperature inside the fire is too high, and our part may simply burn out;
- It is also necessary to ensure that black or blue spots do not appear on the metal product, which indicate deformation of the metal as a result of excessive softening. And if the metal has turned white, then such a part can be safely thrown away.
- As soon as the metal object heats up to the temperature we need, we take it out and first lower it into the oil. We do this three times, the first time for three seconds. Each time we increase the time by the same amount. We lower and take out sharply;
- Next, lower the metal tool into a container of water and leave it there until it cools completely.
Parts or objects that have an elongated shape are placed vertically in the water. To estimate the hardening temperature in a fire, we use a color table. Instead of a fire, you can use any stove.
Features of aluminum hardening
The need to harden any aluminum product at home rarely arises, since all finished products made from casting and wrought alloys usually undergo the required heat treatment and practically do not lose their hardness and rigidity during operation.
A home craftsman may have such a need after welding parts made of aluminum alloys together, since in this case they very often lose their rigidity in the area adjacent to the weld. But it is very difficult to harden aluminum at home, because to do this you need to know exactly the type of alloy and maintain thermal parameters with an accuracy of at least ±5 °C.
Cooling also requires certain skills, because if the technology is not followed accurately, the product may malfunction. If you still want to master this type of heat treatment for use at home, then first of all you need to acquire a furnace with an accurate thermostat, and also be prepared for the fact that each time you will have to harden several samples in turn to select the necessary parameters of the thermal process.
Checking metal for heat treatment
Before we start heating, we need to make sure that the material of the tool we purchased is not heat-treated. We do the test using an ordinary soldering iron. We heat the tool and run it over the metal surface of interest to us. If the soldering iron sticks to the metal, then there can be no talk of any heat treatment. Smooth passage of the soldering iron over the surface of the steel or bouncing off it indicates that the item we are testing is either well heat-treated or processed too much. If there is no heat treatment, we do it ourselves.
Hardening a knife with graphite
Heat treatment of metal with graphite is good when you need to harden not the entire object, but only part of it. For a knife, this is the edge. The sequence of the knife heat treatment process at home:
- We check the tip of the knife for hardness using a needle file. If the metal grinds off easily, and the needle file makes a dull sound, then the knife is not heat-treated;
- for this process you will need graphite, which can be obtained from round batteries, take the leads of a simple pencil, or use the graphite brushes of a generator;
- we turn the mined graphite into powder;
- We use a DC welding machine as a power source. Set to minimum;
- We make the substrate from galvanized sheet. Pour graphite powder onto it;
- We connect the “plus” of the welding device to the substrate, and the “minus” to the knife handle;
- then carefully move the knife blade along the graphite so that it does not touch the substrate. We also make sure that the graphite does not ignite, otherwise our knife will be damaged;
- when the blade moves across the graphite, the latter will produce sparks. As soon as we see that the tip of the knife has heated up, we stop the process. Approximate hardening time: no more than 5 minutes;
- let the knife cool naturally, then take a file and check the hardness. If the sound made by the file upon contact with the knife is loud, and the tip cannot be sharpened, then the hardness of the blade is high.
The hardening process in production is much easier than at home. If necessary, you can try to harden the desired object or tool using “clumsy” methods using improvised means.
Literature and sources used:
- Surface phenomena in metals and alloys / V.K. Semenchenko. - M.: Gostekhizdat
- Ultra-fast hardening of liquid alloys. — Moscow: Mechanical Engineering
- Wikipedia article
Checking the quality of hardening
In order to determine whether a steel product has been hardened to the required hardness, the home craftsman does not have many ways.
The traditional way is to try to scratch the metal with a file (not a diamond one), which usually has a hardness of 55÷60 HRC. If grooves remain on the surface, this means that it was not possible to harden the steel to the required value and its hardness is below this value. If the file slides over the surface of the hardened metal, then its hardness is normal.
Another way to test the quality of home tempering is to scratch the surface of a bottle glass with tempered steel (see photo below). In addition to hardness, at home, if you have certain skills, you can also check the structure of the metal. To do this, it is necessary to harden several samples of the same steel in different modes, and then compare the structure and grain size by eye.