Native copper measuring about 4 cm
Copper
- a mineral from the class of native elements. Fe, Ag, Au, As and other elements are found in natural minerals as impurities or forming solid solutions with Cu. The simple substance copper is a ductile transition metal of golden-pink color (pink in the absence of an oxide film). One of the first metals widely mastered by man due to its relative availability for extraction from ore and low melting point. It is one of the seven metals known to man since very ancient times. Copper is an essential element for all higher plants and animals.
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Gold
– structure and physical properties
Aluminum
– structure and physical properties
What is
Copper is a pinkish metal with a golden-metallic luster. Element No. 29 of the Mendeleev periodic table. International designation: Cu (Cuprum).
Pure metal is soft, so it is often used with impurities. Plastic: stretches to micron diameters.
In air it becomes covered with a film, acquiring a yellowish-red tint. Thin plates are greenish-blue when exposed to light.
According to the official classification, it is classified as a heavy non-ferrous metal. This group also includes lead, zinc, tin, and nickel.
Basic properties of copper
Physical properties.
In air, copper acquires a bright yellowish-red hue due to the formation of an oxide film. Thin plates have a greenish-blue color when examined through them. In its pure form, copper is quite soft, malleable and easily rolled and drawn. Impurities can increase its hardness.
The high electrical conductivity of copper can be called the main property that determines its predominant use. Copper also has very high thermal conductivity. Impurities such as iron, phosphorus, tin, antimony and arsenic affect the basic properties and reduce electrical and thermal conductivity. According to these indicators, copper is second only to silver.
Copper has high densities, melting points and boiling points. An important property is also good resistance to corrosion. For example, at high humidity, iron oxidizes much faster.
Copper lends itself well to processing: it is rolled into copper sheets and copper rods, and drawn into copper wire with a thickness brought to thousandths of a millimeter. This metal is diamagnetic, that is, it is magnetized against the direction of the external magnetic field.
Chemical properties.
Copper is a relatively low-active metal. Under normal conditions in dry air, its oxidation does not occur. It reacts easily with halogens, selenium and sulfur. Acids without oxidizing properties have no effect on copper. There are no chemical reactions with hydrogen, carbon and nitrogen. In humid air, oxidation occurs to form copper (II) carbonate, the top layer of platinum. Copper is amphoteric, meaning it forms cations and anions in the earth's crust. Depending on the conditions, copper compounds exhibit acidic or basic properties.
Story
Copper is one of the first metals that humanity dealt with. This was facilitated by the advantages: high prevalence, availability, relatively low melting point.
People appreciated the benefits of copper eight thousand years ago.
The Copper Age began immediately after the Stone Age:
- Copper artifacts dug up in the territory of modern Turkey are recognized as the oldest. These are beads and decorative overlays.
- Cutting tools and utensils were made from metal.
- The history of the discovery of copper mines in Rus' begins in the Urals two thousand years before the new era. Then there were the Caucasus, Altai, Siberia.
- Industrial processing using bronze began in the 14th century. Cannons and bells were cast from the alloy.
The Tsar Bell and the Tsar Cannon were cast from bronze.
It is assumed that the metal is named after the island of Cyprus. Copper deposits were discovered here back in the 3rd century BC, and the population mastered copper smelting.
M. Vasmer’s “Etymological Dictionary of the Russian Language” links the origin of the Russian term copper with the ancient German root smid – blacksmith, metal.
Reserves, production
Global quantities of copper ore are estimated at one billion tonnes (explored). The presence of half is confirmed. Scientists believe that the earth's crust hides another three billion tons of copper ore.
Native copper
Countries on all continents have rich reserves:
- America – Chile, Canada, USA.
- Asia – Kazakhstan, Iran.
- Africa – South Africa, Zambia, Zaire.
Russia accounts for 3% of world reserves. The deposits are concentrated in the Urals. The main producer is the Norilsk Nickel concern.
Ore is mined by open or closed methods, depending on the depth of occurrence.
The annual global ore production is 15-20 million tons.
Copper ore mining in Russia
The structure of the copper raw material base in Russia differs significantly from the world market. The main share in it falls on sulfide copper-nickel (40%) and pyrite (19%) mines. While in other countries porphyry copper deposits and cuprous sandstones predominate.
Copper ore deposits in Russia
Answering the question of where copper ores are mined in Russia, the Taimyr Autonomous Okrug should first be highlighted. More than 60% of all copper ore deposits in Russia are concentrated in the Oktyabrsky, Tapakhninsky and Norilsk deposits. About one third of the mineral is mined in the Ural copper mining region.
A large Udokan mine has been discovered in the Chita region, which is not yet being developed due to undeveloped transport infrastructure. According to expert data, the exploited deposits in the Russian Federation will last no more than 30 years.
Source
Physico-chemical parameters
Copper is a metal with typical external characteristics (brilliance, smoothness) and crystal lattice structure. Endowed with high electrical and thermal conductivity. According to these physical properties, it is second only to silver.
| Name, symbol, number | Copper/Cuprum (Cu), 29 |
| Atomic mass (molar mass) | 63.546(3)a. e.m. (g/mol) |
| Electronic configuration | [Ar] 3d10 4s1 |
| Atomic radius | 128 pm |
| Chemical properties | |
| Covalent radius | 117 pm |
| Ion radius | (+2e) 73 (+1e) 77 (K=6) rm |
| Electronegativity | 1.90 (Pauling scale) |
| Electrode potential | +0.337 V/ +0.521 V |
| Oxidation states | 3, 2, 1, 0 |
| Ionization energy (first electron) | 745.0 (7.72) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 8.92 g/cm³ |
| Melting temperature | 1356.55 K (1083.4 °C) |
| Boiling temperature | 2567 °C |
| Ud. heat of fusion | 13.01 kJ/mol |
| Ud. heat of vaporization | 304.6 kJ/mol |
| Molar heat capacity | 24.44 J/(K mol) |
| Molar volume | 7.1 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3.615 Å |
| Debye temperature | 315 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 401 W/(m K) |
| CAS number | 7440-50-8 |
The main chemical property of metal assessed by humans is zero corrosion. Copper is chemically inactive and does not oxidize under standard conditions.
Zinc, Zn
Latin name Zincum, chemical symbol Zn. Element of period 4, located in group II, B-subgroup. Serial number 30. Mass - 65.37. Structure of electronic shells: 1s2 2s22p6 3s23p63d10 4s2 (in the ground state). Valency and oxidation state: II(+) and +2 (respectively).
Methods of production in industry:
- Reduction by carbon upon heating: ZnO+ C→ CO↑ + Zn.
- Hydrometallurgy: ZnO + H2SO4 → ZnSO4+ H2O; ZnSO4+ Fe → FeSO4+ Zn↓.
- Electrolysis: zinc is reduced at the cathode Zn2+ + 2H+ + 4ē → Zn↓ + H2.
Zinc is a silver-gray metal ( Fig. 3 ). Solid, conducts heat and electricity. Oxidized by oxygen when heated. Does not interact with boron, carbon, silicon, nitrogen. It does not dissolve in water, but upon strong heating it reacts with water vapor to form zinc oxide and release hydrogen. Reacts with acids other than nitric acid, displacing hydrogen. Displaces metals located to the right in the activity series from solutions of their salts.
Rice. 3. Zinc
table 2
Characteristics of connections
| Substance classes | Names and formulas | Properties |
| Oxides | Zinc oxide, ZnO | Amphoteric. |
| Hydroxides | Zinc hydroxide Zn(OH)2 | Amphoteric. |
Zinc is used as a protective material to prevent rust (galvanizing) of steel and iron products. The metal is used in construction, the production of household appliances and for other purposes.
Copper in nature
In nature, two manifestations of the element have been identified - nuggets and components of compounds with other elements.
Copper nugget
Most often these are compounds: oxides, sulfides, bicarbonates. The most common raw material is copper pyrite.
Copper gives deep blue, cyan, greenish shades to malachite, turquoise, chrysocolla, and other minerals of the jewelry and decorative segment.
Methods of obtaining
The metal content in ores does not exceed 2%. Therefore, they are enriched before smelting. There are two ways to obtain copper: pyro- and hydrometallurgical.
Pyrometallurgical
A multi-level process that includes the following steps:
- Enrichment. Ores are enriched by flotation. Copper particles suspended in water “cling” to air bubbles, which carry them to the surface. The output is a concentrated powder with 12-36% copper.
- Burning. The procedure is indicated for poor (9-24% copper) copper ores and con sulfur. When calcined with oxygen, the proportion of sulfur drops by half.
- Fuse. Shaft or reverberatory type furnaces are loaded with pieces of ore or powder concentrate at 1452°C. Copper matte is obtained.
- Purge. In converters it is exposed to compressed air. Sulfides and iron are oxidized, almost pure (98.51 - 99.51%) blister copper plus iron and other valuable components in trace quantities are formed.
- Refining. The rough product is sent for refining - with flame, then with electrolyte. Impurities are removed with gases. After the first stage, the metal is purified to 99.51%, after the final stage – to 99.96%.
The method is applied to 9/10 of the extracted raw materials.
Hydrometallurgical
It consists of treating raw materials with low concentration dissolved sulfuric acid and isolating the copper metal product.
The method is optimal for ores with a minimum percentage of copper. Removal of other components is not provided.
Varieties of copper ores
There are nine geological types of copper ores of industrial importance:
- Iron-nickel ores occurring in igneous rocks.
- Cuprous sandstones and shales. Stratiform reserves account for 30% of copper reserves and therefore occupy second place in this list.
- Copper-nickel. The deposits are distinguished by a variety of shapes with large inclusions of the desired metal.
- Porphyry copper. They are the undisputed leader and provide 40% of global copper production.
- Carbonatite. They are unique in that there is only one deposit in the world; in addition, they contain alkaline compounds.
- Quartz-sulfide. They do not play a significant role in ensuring production.
- Native. They are located in oxidation areas of copper-sulfide ore mines.
- Skarn. They are located among limestones and are characterized by extreme heterogeneity of morphological structure.
Copper in the listed list of ores can be presented in sulfide, oxide or mixed form, which determines the corresponding types of deposits. Based on their structure in rocks, deposits are divided into disseminated, massive and continuous textures. In the near future, this list may be supplemented by ores lying at the bottom of seas and oceans, as well as nodules of uranium deposits.
Alloys
The range of copper alloys with other components includes dozens of items.
Copper alloys and their applications
They are used more often than pure metal because they reduce the disadvantages inherent in pure metal. That is, they make the product stronger, more stable, and cheaper.
Copper connections are divided into two groups:
- Bronze - with tin.
- Brass – with zinc.
In addition to these main alloying components, the compound contains aluminum, nickel, bismuth, titanium, silver, gold, and non-metallic elements.
Copper roofing
The beautiful color that is characteristic of copper (see photo), as well as its exceptional corrosion resistance, was the reason that many years ago it began to be used as a roofing material. For a long time, copper in this capacity was inferior to cheaper steel and aluminum, but now architects and designers have again paid attention to this unique metal in all respects.
A copper roof with a luxurious color not only looks attractive from an aesthetic point of view and can serve its owners for decades, but is also able to successfully withstand significant mechanical, temperature and any other influences. Houses covered with roofing made of this metal look stylish and presentable.
Copper roofing
Areas of application
The properties of the metal determine its use in various fields. The main consumer is the industrial complex.
Industry
Metal and alloys are used by the following industries:
- Electrical engineering, radio electronics. Cables (power, others), wires. Winding in transformers. Heat exchange devices (heating radiators, air conditioners, computer coolers, laptop heat pipes).
- Instrumentation, mechanical engineering. Parts and machine components are made from alloys of copper with zinc, tin, and aluminum. Without it, it is impossible to create galvanic cells and batteries.
- Pipes. For transporting steam, water, gas. In the energy sector, shipbuilding, for domestic needs.
Copper heat pipe cooling system in a laptop
In Japan, copper pipelines are recognized as earthquake-resistant, which is vital for this country.
Copper pipes
Construction
Roofs made of copper sheets are environmentally friendly; they do not need to be painted, since moisture and weather disasters are not scary. Service life – up to 100 years.
Medicine
The characteristics of the metal as an antiseptic and astringent are in demand in medicine.
It is a component of eye drops and mixtures for the treatment of burns.
Copper door handles and other surfaces are an attribute of medical institutions.
Copper compounds suppress the swine flu virus.
Jewelry
Jewelers use copper-based alloys.
Copper ring
Red or rose gold is a conglomerate of the noble metal with copper.
Its amount in the composition determines the final shade:
- 25% – pink;
- 50% – red.
These types of gold are the most beloved by jewelers. Copper makes products stronger, while simultaneously reducing the cost.
The second popular jewelry alloy is cupronickel (copper + nickel).
Other industries
- Copper oxide is the basis of cuprate, used in superconductors.
- Brass is used to make cartridges for rifles and artillery.
- Nickel silver is used to mint coins, create interior decorations, and cutlery.
- Copper is involved in the synthesis of chlorophyll. It is always added to mineral fertilizers for plants.
Copper
(Cu from Latin Cuprum) is an element of the eleventh group of the fourth period (a side subgroup of the first group) of the periodic system of chemical elements of D. I. Mendeleev, with atomic number 29. The simple substance copper is a plastic transition metal of golden-pink color (pink color when absence of oxide film). It has been widely used by humans for a long time. Copper coin (photo)
History
Copper is one of the first metals well mastered by man due to its availability from ore and low melting point. This metal is found in nature in native form more often than gold, silver and iron. Some of the most ancient copper products, as well as slag - evidence of its smelting from ores - were found in Turkey, during excavations of the settlement of Çatalhöyük. The Copper Age, when copper objects became widespread, follows the Stone Age in world history. Experimental studies by S. A. Semenov and his colleagues showed that, despite the softness of copper, copper tools, compared to stone ones, provide a significant advantage in the speed of cutting, planing, drilling and sawing wood, and processing bone takes approximately the same time as for stone tools
In ancient times, copper was also used in the form of an alloy with tin - bronze - for the manufacture of weapons, etc., the Bronze Age replaced the Copper Age. An alloy of copper and tin (bronze) was produced for the first time in 3000 BC. e. in the Middle East. Bronze attracted people because of its strength and good malleability, which made it suitable for making labor and hunting tools, dishes, and jewelry. All these items are found in archaeological excavations. The Bronze Age regarding tools was replaced by the Iron Age.
Copper was originally mined from malachite ore rather than sulfide ore, as it does not require pre-roasting. To do this, a mixture of ore and coal was placed in a clay vessel, the vessel was placed in a small pit, and the mixture was set on fire. The released carbon monoxide reduced malachite to free copper:
In Cyprus, already in the 3rd millennium BC there were copper mines and copper smelting was carried out.
Copper mines appeared on the territory of Russia and neighboring countries two millennia BC. e. Their remains are found in the Urals (the most famous deposit is Kargaly), in Transcaucasia, in Siberia, Altai, and in Ukraine.
In the XIII-XIV centuries. mastered industrial copper smelting. In Moscow in the 15th century. The Cannon Yard was founded, where guns of various calibers were cast from bronze. A lot of copper was used to make bells. Such works of casting art as the Tsar Cannon (1586), the Tsar Bell (1735), the Bronze Horseman (1782) were cast from bronze, and a statue of the Big Buddha was cast in Japan (Todai-ji Temple) ( 752).
With the discovery of electricity in the 18th-19th centuries. large volumes of copper began to be used for the production of wires and other related products. And although in the 20th century. wires often began to be made of aluminum; copper has not lost its importance in electrical engineering.
Origin of the name copper
The Latin name for copper Cuprum (ancient Aes cuprium, Aes cyprium) comes from the name of the island of Cyprus, where there was a rich deposit.
Strabo calls copper chalkos, from the name of the city of Chalkis on Euboea. From this word came many ancient Greek names for copper and bronze objects, blacksmithing, blacksmithing and casting. The second Latin name for copper Aes (Sanskrit ayas, Gothic aiz, German erz, English ore) means ore or mine.
The words copper and copper are found in the most ancient Russian literary monuments. Slavic *mědь “copper” does not have a clear etymology, perhaps an original word. V.I. Abaev assumed the origin of the word from the name of the country Media: *Copper from Ir. Māda - through the Greek. Μηδία[8]. According to the etymology of M. Vasmer, the word “copper” is related to ancient German. smid “blacksmith”, smîda “metal”.
Copper was designated by the alchemical symbol “♀” - “mirror of Venus”, and sometimes copper itself was also called “Venus” by alchemists. This is due to the fact that the goddess of beauty, Venus (Aphrodite), was the goddess of Cyprus, and mirrors were made from copper. This symbol of Venus was also depicted on the brand of the Polevsky copper smelter, it was used to brand Polevsky copper from 1735 to 1759, and is depicted on the modern coat of arms of the city of Polevskoy. Polevsky’s Gumeshevsky mine, the largest copper ore deposit of the Russian Empire in the Middle Urals in the 18th-19th centuries, is associated with a famous character in P. P. Bazhov’s fairy tales - the Mistress of the Copper Mountain, the patroness of the mining of malachite and copper. According to one hypothesis, she is the image of the goddess Venus refracted by the popular consciousness.
Copper in nature
The average copper content in the earth's crust (clarke) is (4.7-5.5) 10−3% (by mass). In sea and river water the copper content is much lower: 3·10−7% and 10−7% (by weight), respectively.
Copper occurs in nature both in compounds and in native form. Of industrial importance are chalcopyrite CuFeS2, also known as copper pyrite, chalcocite Cu2S and bornite Cu5FeS4. Together with them, other copper minerals are also found: covellite CuS, cuprite Cu2O, azurite Cu3(CO3)2(OH)2, malachite Cu2CO3(OH)2. Sometimes copper is found in native form; the mass of individual clusters can reach 400 tons. Copper sulfides are formed mainly in medium-temperature hydrothermal veins. Copper deposits are also often found in sedimentary rocks - cuprous sandstones and shales. The most famous deposits of this type are Udokan in the Trans-Baikal Territory, Zhezkazgan in Kazakhstan, the copper belt of Central Africa and Mansfeld in Germany. The other richest copper deposits are in Chile (Escondida and Colhausi) and the USA (Morenci).
Most copper ore is mined by open pit mining. The copper content in the ore ranges from 0.3 to 1.0%.
Physical properties of copper
Copper is a golden-pink ductile metal; in air it quickly becomes covered with an oxide film, which gives it a characteristic intense yellowish-red hue. Thin films of copper have a greenish-blue color when exposed to light.
Along with osmium, cesium and gold, copper is one of the four metals that have a distinct coloration that is different from the gray or silver of other metals. This color tint is explained by the presence of electronic transitions between the filled third and half-empty fourth atomic orbitals: the energy difference between them corresponds to the wavelength of orange light. The same mechanism is responsible for the characteristic color of gold.
Copper forms a cubic face-centered lattice, space group F m3m, a = 0.36150 nm, Z = 4.
Copper has high thermal and electrical conductivity (it ranks second in electrical conductivity among metals after silver). Specific electrical conductivity at 20 °C: 55.5-58 MS/m. Copper has a relatively large temperature coefficient of resistance: 0.4%/°C and is weakly dependent on temperature over a wide temperature range. Copper is diamagnetic.
There are a number of copper alloys: brass - with zinc, bronze - with tin and other elements, cupronickel - with nickel and others.
Atomic density of copper (N0) = 8.52 * 10 28 (atom/m³).
Application of copper
in electrical engineering
Due to its low resistivity (second only to silver, resistivity at 20 °C: 0.01724-0.0180 μOhm m/), copper is widely used in electrical engineering for the manufacture of power and other cables, wires or other conductors, for example, in printed circuit wiring. Copper wires, in turn, are also used in the windings of electric drives (household: electric motors) and power transformers. For these purposes, the metal must be very pure: impurities sharply reduce electrical conductivity. For example, the presence of 0.02% aluminum in copper reduces its electrical conductivity by almost 10%.
Heat exchange
Cooling system made of copper on heat pipes in a laptop Another useful quality of copper is its high thermal conductivity. This allows it to be used in various heat sink devices, heat exchangers, which include well-known cooling, air conditioning and heating radiators, computer coolers, and heat pipes.
For pipe production
Due to their high mechanical strength and suitability for machining, copper seamless round pipes are widely used for transporting liquids and gases: in internal water supply systems, heating systems, gas supply systems, air conditioning systems and refrigeration units. In a number of countries, copper pipes are the main material used for these purposes: in France, Great Britain and Australia for gas supply to buildings, in Great Britain, USA, Sweden and Hong Kong for water supply, in Great Britain and Sweden for heating.
Copper rods (photo)
Copper pipes (photo)
In Russia, the production of water and gas pipes from copper is standardized by the national standard GOST R 52318-2005, and the application in this capacity by the federal Code of Rules SP 40-108-2004. In addition, pipelines made of copper and copper alloys are widely used in the shipbuilding and energy industries to transport liquids and steam.
Copper alloys
Alloys using copper are widely used in various fields of technology, the most widespread of which are the above-mentioned bronze and brass. Both alloys are general names for a whole family of materials, which, in addition to tin and zinc, may include nickel, bismuth and other metals. For example, the composition of gun bronze, used for the manufacture of artillery pieces until the 19th century, includes all three main metals - copper, tin, zinc; the recipe changed depending on the time and place of manufacture of the weapon. A large amount of brass is used for the manufacture of artillery ammunition casings and weapon casings, due to its manufacturability and high ductility. For machine parts, alloys of copper with zinc, tin, aluminum, silicon, etc. (rather than pure copper) are used because of their greater strength: 30-40 kgf/mm² for alloys and 25-29 kgf/mm² for technically pure copper. Copper alloys (except beryllium bronze and some aluminum bronzes) do not change their mechanical properties during heat treatment, and their mechanical properties and wear resistance are determined only by the chemical composition and its effect on the structure. Modulus of elasticity of copper alloys (900-12000 kgf/mm², lower than that of steel). The main advantage of copper alloys is their low coefficient of friction (which makes them especially rational for use in sliding pairs), combined for many alloys with high ductility and good resistance to corrosion in a number of aggressive environments (copper-nickel alloys and aluminum bronzes) and good electrical conductivity. The magnitude of the coefficient of friction is almost the same for all copper alloys, while the mechanical properties and wear resistance, as well as behavior under corrosion conditions, depend on the composition of the alloys, and therefore on the structure. Strength is higher in two-phase alloys, and ductility is higher in single-phase alloys. Copper-nickel alloy (cupronickel) is used for minting small change coins. Copper-nickel alloys, including the so-called “Admiralty” alloy, are widely used in shipbuilding (turbine exhaust steam condenser tubes cooled by sea water) and applications related to the possibility of aggressive action of sea water due to their high corrosion resistance. Copper is an important component of hard solders - alloys with a melting point of 590-880 ° C, which have good adhesion to most metals, and are used for durable connections of a variety of metal parts, especially dissimilar metals, from pipeline fittings to liquid rocket engines.
Alloys in which copper is significant
A duralumin part of the Hindenburg airship (LZ 129) damaged by fire. Dural (duralumin) is defined as an alloy of aluminum and copper (copper in duralumin is 4.4%).
Jewelry alloys
In jewelry, alloys of copper and gold are often used to increase the resistance of products to deformation and abrasion, since pure gold is a very soft metal and is not resistant to mechanical stress.
Copper connections
Copper oxides are used to produce yttrium-barium-copper oxide (cuprate) YBa2Cu3O7-δ, which is the basis for the production of high-temperature superconductors. Copper is used to produce copper oxide galvanic cells and batteries.
Other Applications
Copper is the most widely used acetylene polymerization catalyst. Due to the fact that copper is a catalyst for the polymerization of acetylene (forms compounds of copper with acetylene), copper pipelines for transporting acetylene can only be used if the copper content in the alloy of the pipe material is no more than 64%.
Copper is widely used in architecture. Roofs and facades made of thin sheet copper, due to the auto-attenuation of the corrosion process of the copper sheet, serve trouble-free for 100-150 years. In Russia, the use of copper sheets for roofs and facades is regulated by the federal Code of Rules SP 31-116-2006.
The predicted new mass use of copper promises to be its use as bactericidal surfaces in medical institutions to reduce intra-hospital bacterial transfer: doors, handles, water stop valves, railings, bed rails, table tops - all surfaces touched by the human hand.
Copper vapor is used as a working fluid in copper vapor lasers at lasing wavelengths of 510 and 578 nm.
Copper production and mining.
World copper production in 2000 was about 15 million tons, and in 2004 - about 14 million tons. World reserves in 2000 were, according to experts, 954 million tons, of which 687 million tons were proven reserves; Russia accounted for 3.2% of total and 3.1% of confirmed world reserves. Thus, at the current rate of consumption, copper reserves will last approximately 60 years.
Production of refined copper in Russia in 2006 amounted to 881.2 thousand tons, consumption - 591.4 thousand tons. The main copper producers in Russia were:
Company thousand tons % Norilsk Nickel 425 45% Uralelectromed 351 37% Russian Copper Company 166 18%
In 2009, Metalloinvest Holding joined these copper producers in Russia, purchasing the rights to develop the new Udokanskoye copper deposit. World copper production in 2007 was 15.4 million tons, and in 2008 - 15.7 million tons.
The leaders in production were:
Chile (5.560 million tons in 2007 and 5.600 million tons in 2008), USA (1.170/1.310), Peru (1.190/1.220), China (0.946/1.000), Australia (0.870/0.850), Russia (0.740 /0.750), Indonesia (0.797/0.650), Canada (0.589/0.590), Zambia (0.520/0.560), Kazakhstan (0.407/0.460), Poland (0.452/0.430), Mexico (0.347/0.270). In terms of global production and consumption, copper ranks third after iron and aluminum.
The world's explored copper reserves at the end of 2008 amounted to 1 billion tons, of which 550 million tons were confirmed. Moreover, it is estimated that the global world reserves on land amount to 3 billion tons, and deep-sea resources are estimated at 700 million tons.
Copper powder (photo)
Modern mining methods
More than 170 minerals containing copper are now known, but only 14-15 of them are of industrial importance. This is chalcopyrite (also known as copper pyrite), malachite, and native copper is also found. Copper ores often contain molybdenum, nickel, lead, cobalt as impurities, and less commonly gold and silver. Typically, copper ores are beneficiated in factories before being sent to copper smelters. Kazakhstan, the USA, Chile, Canada, and African countries are rich in copper - Zaire, Zambia, South Africa. Escondida is the world's largest copper ore quarry (located in Chile). Depending on the depth of occurrence, ore is mined using open or closed methods.
90% of primary copper is obtained by pyrometallurgical method, 10% by hydrometallurgical method. The hydrometallurgical method is the production of copper by dissolving it in a weak solution of sulfuric acid and subsequent separation of metallic (blister) copper from the solution. The pyrometallurgical method consists of several stages: enrichment, roasting, smelting for matte, purging in a converter, refining.
To enrich copper ores, the flotation method is used (based on the use of different wettability of copper-containing particles and waste rock), which allows one to obtain copper concentrate containing from 10 to 35% copper.
Copper ores and concentrates with high sulfur content are subjected to oxidative roasting. In the process of heating the concentrate or ore to 700-800 °C in the presence of atmospheric oxygen, sulfides are oxidized and the sulfur content is reduced by almost half of the original. Only poor concentrates (with a copper content of 8 to 25%) are fired, and rich concentrates (from 25 to 35% copper) are melted without firing.
After roasting, the ore and copper concentrate are smelted into matte, which is an alloy containing copper and iron sulfides. Matte contains from 30 to 50% copper, 20-40% iron, 22-25% sulfur, in addition, matte contains impurities of nickel, zinc, lead, gold, and silver. Most often, smelting is carried out in fiery reverberatory furnaces. The temperature in the melting zone is 1450 °C.
In order to oxidize sulfides and iron, the resulting copper matte is subjected to blowing with compressed air in horizontal converters with side blast. The resulting oxides are converted into slag. The temperature in the converter is 1200–1300 °C. Interestingly, heat is released in the converter due to chemical reactions, without fuel supply. Thus, the converter produces blister copper containing 98.4–99.4% copper, 0.01–0.04% iron, 0.02–0.1% sulfur and a small amount of nickel, tin, antimony, silver, gold. This copper is poured into a ladle and poured into steel molds or a casting machine.
Next, to remove harmful impurities, blister copper is refined (fire refining and then electrolytic refining are carried out). The essence of fire refining of blister copper is the oxidation of impurities, removing them with gases and converting them into slag. After fire refining, copper with a purity of 99.0–99.7% is obtained. It is poured into molds and ingots are obtained for further smelting of alloys (bronze and brass) or ingots for electrolytic refining.
Electrolytic refining is carried out to obtain pure copper (99.95%). Electrolysis is carried out in baths where the anode is made of fire-refined copper, and the cathode is made of thin sheets of pure copper. The electrolyte is a solution of sulfuric acid with copper sulfate. During electrolysis, the concentration of sulfuric acid increases. When a direct current is passed, the anode dissolves, the copper goes into solution, and, cleaned of impurities, is deposited on the cathodes. Impurities settle to the bottom of the bath in the form of sludge, which is processed to extract valuable metals. When receiving 1000 tons of electrolytic copper, you can get up to 3 kg of silver and 200 g of gold. The cathodes are unloaded after 5-12 days, when their weight reaches 60 to 90 kg. They are thoroughly washed and then melted in electric furnaces.
Copper granules (photo)
Meaning for humans
Copper is inherent in the human body initially:
- Participates in the formation of red blood cells, collagen, elastin.
- Activates the endocrine system, slows down the aging of the body.
- Its deficiency is fraught with a slowdown in protein metabolism. This leads to pathologies in the development of the skeleton and blood composition.
It is found in many foods. Beef liver, oysters, sesame seeds, cocoa powder, black pepper, and buckwheat are rich in copper. And also nuts (hazelnuts, walnuts, cashews, peanuts, almonds).
Warning
The metal contains isotopes: two stable ones plus two dozen unstable ones. Although the half-life of the “long-liver” is less than 2.5 days, the material is toxic.
Therefore, the use of copper is controlled.
In Russia at the federal level (national standard, federal Code of Rules) the following is regulated:
- Production and use of copper water, steam and gas pipes.
- Amount of copper in drinking water.
1 liter of drinking water should not contain more than 1 mg of copper.
Excess copper components cause poisoning of the body. Copper utensils are not suitable for cooking.
A quarry in which copper ore was extracted by open pit mining becomes a source of toxic compounds.
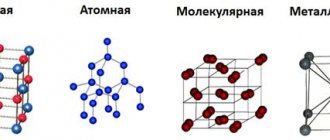
Composition and structure
Copper is a combination of a huge number of crystals of silver, calcium, gold, lead, and nickel. The metals that cuprum consists of are distinguished by ease of processing and relative ductility.
The unit cell of the structural lattice is cubic in shape. Each cell represents a compound of 4 atoms.
During mining, the ore is saturated with a huge amount of impurities. They affect the technical characteristics of the remelted metal and its structure. Common impurities:
- Oxygen is an impurity, the content of which in the composition can reach 0.008%. When exposed to high temperatures, the oxygen content quickly decreases.
- Bismuth is a component that negatively affects the technical characteristics of the finished metal. The permissible amount in the composition is up to 0.001%.
- Manganese has virtually no effect on the properties of cuprum.
- Nickel - reduces thermal conductivity.
- Arsenic - does not affect the properties of the remelted metal. Arsenic neutralizes the negative effects of bismuth, oxygen, and antimony on the final material.
- Tin - enhances thermal conductivity.
- Antimony - reduces thermal and electrical conductivity. Permissible content in the composition is up to 0.05%.
- Sulfur, selenium - reduce the plasticity index if their amount in the composition exceeds 0.001%.
- Zinc has virtually no effect on physical and chemical properties.
- Phosphorus is the main deoxidizing agent. Improves mechanical properties.
The percentage of impurities during production may decrease or increase.
Copper ore
Prices
The world price of copper is set on the London Metal Exchange. It depends on demand, determined by the state of the economy.
And fluctuates accordingly:
- By the beginning of 2008, the psychological mark of $8,000 per ton was overcome.
- Six months later it was already $+940, which became a record in the entire history of the exchange.
- At the beginning of 2011, the level was set at $10,000.
Then there was a recession. For 2022, a ton of copper is trading at $8,057. The economic slowdown due to the coronavirus pandemic has had an impact.