It is difficult to imagine modern construction, technology, mechanical engineering and other important industries without the use of the main metal alloys of steel and cast iron. Their production exceeds all others by tens of times.
If we consider steel and cast iron from the point of view of such a science as metallurgy, then the central figure is the state diagram of iron-carbon alloys, which allows us to obtain detailed ideas about the composition and structural transformations in these materials. And also get acquainted with their phase composition.
History of discovery
For the first time, the great metallurgist and inventor Dmitry Konstantinovich Chernov (1868) pointed out that there are certain (special) points in alloys (steels and cast irons). It was he who made an important discovery about polymorphic transformations and is one of the creators of the iron-carbon phase diagram. According to Chernov, the position of these points on the diagram is directly dependent on the percentage of carbon content.
And what is most interesting is that it is from the moment of this discovery that the science of metallography begins its life.
The diagram of iron-carbon alloys is the result of the painstaking work of scientists from several countries around the world. All letter designations of the main points and phases in the diagram are international.
State diagram [edit | edit code ]
Iron forms the chemical compound Fe3C cementite with carbon. Since in practice iron-based metal alloys with a carbon content of up to 5% are used, the part of the phase diagram from pure iron to cementite is of practical interest. Since cementite is a metastable phase, the corresponding diagram is called metastable
(solid lines in the figure).
For gray cast irons and graphitized steels, stable
part of the iron-graphite (Fe-Gr) diagram, since it is graphite that is the stable phase in this case. Cementite is released from the melt much faster than graphite and can exist in many steels and white cast irons for quite a long time, despite its metastability. In gray cast irons, graphite necessarily exists.
In the figure, thin dotted lines show the lines of stable equilibrium (that is, with the participation of graphite), where they differ from the lines of metastable equilibrium (with the participation of cementite), and the corresponding points are indicated by a dash. The phase and point designations in this diagram are based on informal international agreement.
Elements of the iron-carbon diagram
Brief information about each of these elements.
Iron is a silvery-gray metal. Specific gravity - 7.86 g/cm3. It has a melting point of 1539° C.
When iron and other metals interact, compounds called substitution solutions are formed. If with non-metals, for example with carbon or hydrogen, then - interstitial solutions.
Iron has the ability, being initially solid, to exist in several states, which in metallurgy are usually called “alpha” and “gamma”. This quality is called polymorphism. More on this later in the article.
Carbon is a non-metal. If it acts as graphite, then the melting point is 3500° C. If it is like diamond, it is 5000° C. The density of carbon is 2.5 g/cm3. It also has polymorphic properties.
In iron-carbon alloys, this element forms a solid solution that contains a ferrum called cementite (Fe3C). Also forms graphite in cast irons.
Chemical properties
As a chemical compound, cementite has its own physical, chemical and mechanical characteristics. It has a gray crystalline appearance at fracture and is relatively hard with high thermal stability. The main chemical properties of cementite are expressed in the following indicators:
- chemical formula Fe3C;
- decomposition of the structure occurs at temperatures above 1650°C;
- exposed to various acids (especially highly concentrated ones);
- quickly reacts with oxygen.
Based on the existing chemical properties, physical and mechanical properties are formed. The main physical properties include:
- melting point is 1700 °C;
- molecular weight is 179.55 amu;
- The density of cementite is 7.7 g/cm3 at a temperature of 20 °C.
The main mechanical properties include:
- hardness;
- resistance to impact (fragility);
- fracture resistance;
- plastic.
The hardness of this compound reaches high values and is equal to HB 8000 MPa or HRC 70. However, it has sufficient brittleness and low ductility.
Possessing the listed properties, cementite is actively used in the production of cast parts for various purposes. The formation of various types of cementite and its compounds with other forms leads to a change in the characteristics of the resulting steel or cast iron, therefore, to an improvement or decrease in individual consumer properties.
For example, to obtain white cast iron and give it high strength and ductility, they try to convert cementite into graphite. This is achieved during the annealing operation. As the temperature increases, it breaks down into two components: ferrite and graphite.
Depending on the required properties, they try to maintain the required amount of cementite in cast iron. This is especially true for the so-called free fraction of this compound. To reduce its concentration, various methods of chemical and thermal treatment are used. To solve this problem, use a solution of nitric acid in pure alcohol. Structurally free cementite precipitates as a result of boiling a cast iron pig in this solution. In addition, three types of processing are used: annealing, normalizing and hardening.
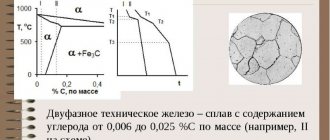
Technical iron contains tertiary cementite in combination with ferrite. It appears along the ferrite boundary at carbon contents from 0.01% to 0.025%. To improve the quality of steel, they try to reduce the content of free cementite. Its concentration is especially observed in soft steel grades. The content of this mixture and perlite per unit volume has a great influence on the quality of stamping. Excessive presence of tertiary cementite, especially in the form of a long chain or network, leads to the formation of breaks during stamping. Therefore, to obtain good forging steel, they try to reduce the amount of tertiary cementite. The structure of such formations should not exceed the second point on the established scale. The resulting hardness should not exceed HB 50 units.
Iron-carbon alloy diagram
As a result of the interaction of the components of the diagram with each other, cementite is obtained - a chemical compound.
As a rule, when studying a diagram by metallurgical students, all stable connections are considered as components, and the graphic image itself is examined in parts.
Also in class, a cooling curve is plotted using the iron-carbon diagram: the percentage of carbon is selected, and then it is necessary to determine which phase corresponds to which temperature on the diagram.
To do this, in addition to the diagram itself, it is necessary to draw a coordinate system (temperature-time). And starting from the maximum degrees, move gradually downward, depicting the curve and areas of transition from one phase to another. In this case, it is necessary to name them and indicate the type of crystal lattice.
Next, let's take a closer look at the graphical representation of the iron-carbon phase diagram itself.
Firstly, it has two forms (parts):
- iron cementite;
- iron-graphite.
Secondly, alloys in which the main “actors” are ferrum and carbon are conventionally divided into:
- become;
- cast irons.
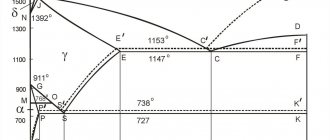
If the carbon in the alloy is less than or equal to 2.14% (point E on the diagram), then it is steel, if more than 2.14% it is cast iron. For this reason, the diagram is divided into two phases.
Lecture No. 3. Iron-carbon alloys
Lecture No. 3. Iron-carbon alloys
Iron-carbon alloys (steels and cast irons) are the most common materials. These are called ferrous metals and make up about 95% of metal production. The state diagram of iron-carbon alloys gives an idea of the structure of steels and cast irons.
3.1. Iron–carbon phase diagram
Before considering transformations in the alloys of this system, let us consider the properties and structure of the components and phases of the system, as well as the areas of their existence.
Components of the Fe–C system
.
Pure iron (Fe)
is a silvery-white polymorphic metal, with a density γ = 7.86 g/cm3, atomic number 26, atomic weight 55.85, melting point 1539°C, and has a low hardness HB80. When heated, iron undergoes polymorphic transformations (Fig. 1.9) and has two polymorphic modifications Feα and Feγ.
Carbon (C)
– non-metallic polymorphic element (graphite and diamond), with a density of 2.25 g/cm3.
Phases of the Fe–C system
. All alloys are combined from three single-phase and two two-phase structural components.
Single-phase structural components
.
Ferrite
is a solid solution of carbon intercalation in Fα, designated Feα(C) – F. The maximum solubility of carbon in ferrite reaches 0.02% at 727°C and 0.006% at 20°C. Ferrite is white crystals; its properties are close to those of commercially pure iron. The region of existence of ferrite is QPG.
Austenite
– solid solution of carbon intercalation in Feγ. It is designated Feγ(C) – A. It dissolves carbon well, at t = 1147°C it contains 2.14% C, and at = 727°C – 0.8% C. Austenite is a paramagnetic, plastic phase. Austenite region NJESG.
Cementite
(C) - chemical compound Fe3C - iron carbide, formed at a carbon content of 6.67%. Melting point 1600°C. It has a white, shiny color, brittle, hard. Can be primary, secondary, tertiary. DFKL cementite area.
There is also a liquid phase located above the liquidus line. Iron dissolves carbon well, forming a homogeneous liquid phase - F.
Two-phase structural components
.
Ledeburite
is a eutectic of the Fe – Fe3C system, it is a mechanical mixture of cementite and austenite and contains 4.3% C. Crystallizes at t = 1147°C, denoted by L. Eutectic reaction: JC↔LC(AE + CF). At a temperature of 727°C, the eutectoid transformation of ledeburite occurs, and its structure will be LS(PS + C)
Perlite –
The eutectoid of the Fe system - Fe3C - is a mechanical mixture consisting of small plates of cementite in a ferrite base. It is designated as P. Pearlite is formed from a single-phase solution of Feγ(C) during the polymorphic transformation Feγ→ Feα at a temperature t = 727°C and a carbon concentration of 0.8%.
Feγ(C)0.8%→ Fe3C6.67% + Feα(C)0.025%
Iron, interacting with carbon, forms a number of chemical compounds: Fe3C, Fe2C, FeC, etc. Since a chemical compound in phase diagrams can be considered as a component, the iron-carbon diagram is usually depicted only up to a carbon content of 6.67%, at which it is formed iron carbide Fe3C (stable chemical compound). Since only this part of the iron-carbon diagram is of practical importance, this part of the diagram is called the iron-cementite phase diagram.
Rice. 3.1. Iron-carbon phase diagram (iron-cementite)
Line ABCD is the liquidus line
(S), line AHJECD -
solidus line
(L) of the iron-cementite system. Along the ECF line, at t = 1147°C, the eutectic transformation AE+ CF → LC occurs, i.e., a eutectic—ledeburite—is formed.
Along the PSK line, the eutectoid transformation FP + CK → PS occurs, that is, the eutectoid of the system is formed - pearlite. Austenite is suitable for the PSK line with a concentration of 0.8% C. If the carbon concentration is less than 0.8%, then ferrite is precipitated from austenite along the GS line; if it is higher, then secondary cementite, CII, is precipitated along the ES line. Line PQ is a line of variable solubility of carbon in the ferrite lattice. Excess carbon forms with iron the chemical compound Fe3C - cementite. To note the peculiarities of the release of cementite in alloys with a carbon concentration of less than 0.025%, it is designated tertiary cementite - CIII. It is released in the form of dispersed inclusions in ferrite grains, increasing its strength.
The temperatures at which phase and structural transformations occur in alloys of the Fe – Fe3C system, i.e. critical points, have a symbol. All critical points are designated by the letter A. When heated, the letter “c” is added to A, that is, Ac, and when cooled, “r” is added, that is, Ar.
The first critical point A1 lies on the PSK line (727°C) and corresponds to the transformation P ↔ A; A2 corresponds to a temperature of 768°C - the Curie point, A3 - the GS line along which the F↔A transformation occurs, the temperature of which depends on the carbon concentration in the alloy, Аcm - the SE line - the beginning of the release of CII.
All alloys of the Fe-Fe3C system are divided into two large groups according to their structural characteristics: steels and cast irons.
Carbon steels
are called alloys of iron and carbon up to a concentration of 2.14% C. This is a theoretical definition. In practice, steels generally do not contain more than 1.5% carbon. They are divided into: hypoeutectoid steels - (containing from 0.025% to 0.8% C, F + P), eutectoid - (0.8% C, P), hypereutectoid - (0.8%...2.14% C, P + CII), Fig. 3.2.
a B C
Rice. 3.2. Microstructures of carbon steels:
a – hypoeutectoid; b – eutectoid; c – hypereutectoid
In hypoeutectoid steel, ferrite is revealed in the microstructure in the form of light fields, and pearlite - in the form of fields of a banded (dark) structure (Fig. 3.2a), where the general light background is ferrite, and the dark places are shadows from protruding cementite plates.
The amount of pearlite in the steel structure increases in proportion to the increase in carbon content, this occurs up to a carbon content of 0.8%, when it becomes the only structural component of eutectoid steel (Fig. 3.2b).
The microstructure of hypereutectoid steel consists of pearlite and secondary cementite, which, upon slow cooling, precipitates in the form of a network along the boundaries of pearlite grains (Fig. 3.2c).
Alloys of iron and carbon containing more than 2.14% carbon (up to 6.67%) are called cast iron
. They are divided into hypoeutectic (2.14%...4.3% C, P + CII+ L(P + C)), eutectic (4.3% C, L(P + C)) and hypereutectic (4.3% C …6.67%C, CI+ L(P + C)) (Fig. 3.3).
a B C
Rice. 3.3. Microstructures of cast iron:
a – hypoeutectic; b – eutectic; c – hypereutectic
In addition, technically pure iron is isolated (up to 0.025% C, F + CIII).
When iron-carbon alloys are cooled, carbon can not only react chemically with iron, but also be released in the form of graphite. In other words, a liquid solution, ferrite and austenite can be in equilibrium not only with cementite, but also with graphite, and then the phase diagram will be iron - graphite.
Cementite is a less stable phase than graphite G, therefore the Fe – Fe3C diagram is unstable, metastable, and Fe – G is stable. The process of graphite formation is called graphitization. Graphitization is more pronounced on the right side of the diagram and leads to the formation of half- or gray cast irons, which have graphite in their structure, in contrast to white cast irons, which contain only cementite in their structure.
3.2. Carbon and permanent impurities in steel, their effect on its properties
The phase composition of any steel in an equilibrium state is ferrite + cementite. The amount of cementite increases in proportion to the increase in carbon content, and since cementite is a hard, brittle phase, the strength properties of steel increase (up to 0.9% C), hardness, and ductility and impact strength decrease. With an increase in carbon content, technological properties deteriorate - weldability, machinability, and deformability in hot and cold states decrease. For every 0.1% C, the cold brittleness threshold increases by 20°C. In addition to iron and carbon, steel always contains permanent impurities.
To permanent
impurities include manganese - Mn, silicon - Si, sulfur - S, phosphorus - P.
Silicon and manganese are technological impurities and are found in carbon steels in amounts of 0.35...0.40% and 0.5...0.8%, respectively. By deoxidizing steel, Si and Mn improve its properties and are useful impurities. Dissolving in ferrite, Si and Mn strengthen it, increase its elastic limit, and Mn binds sulfur and paralyzes its harmful effects.
Sulfur sharply worsens the properties of steel; above the permissible limit (0.06%) it is capable of forming a low-melting eutectic FeS + Fe with iron and causing red brittleness.
Phosphorus is allowed up to 0.045%, dissolving in ferrite, strengthening it and making it brittle at low temperatures - sharply increasing the threshold of cold brittleness. Sulfur and phosphorus are harmful impurities.
In addition to permanent impurities, iron-carbon alloys contain hidden and random impurities.
To the hidden
Impurities include gases (O2, H2, N2) found in steels in very small quantities. These impurities impair the ductility of steel.
Random
are impurities of non-ferrous metals (Cu, Pb, Sn, Sb, etc.) introduced into steel along with charge materials.
3.3. Classification and marking of steels
By chemical composition
steels can be carbon and alloy. Carbon steels contain iron, carbon and impurities, while alloy steels contain additional alloying elements introduced into the steel to change its properties.
By carbon content
Carbon and alloy steels are divided into low-carbon (up to 0.25% C), medium-carbon (0.25...0.7% C) and high-carbon (more than 0.7% C).
By purpose
A distinction is made between structural steels, used for the manufacture of structures, structures, machine parts, and tool steels, used for the manufacture of various tools.
By quality
Structural steels are classified into ordinary quality and high-quality steel. The quality of steel is determined by a set of properties determined by the production process, chemical composition containing gases and harmful impurities. In accordance with GOST, ordinary quality steel should contain no more than 0.04% P and 0.05% S, and high-quality steel should contain no more than 0.035% P and 0.04% S.
Tool carbon steels can be of high quality and high quality (P, S ≤0.035%).
By deoxidation -
depending on the degree of deoxidation during steel smelting, they can be calm (sp), semi-quiet (ps) and boiling (kp), which is indicated in the brand.
By smelting –
converter, open-hearth, electric steels.
Carbon steels.
Carbon structural steels of ordinary quality, depending on the purpose and guaranteed properties, are divided into three groups A, B and C.
Group A steels have guaranteed mechanical properties. They are used as delivered without hot processing. They are marked with the letters Art. and numbers indicating the serial number of the brand. Seven steel grades of this group are produced: St.0, St.1, St.2…St.6. Depending on the deoxidation, the letters “sp”, “ps”, “kp” are placed. For example, St.1sp, St.3kp, St.5ps. As the steel number increases, the carbon content increases (from 0.1 to 0.5% C, with the exception of St.0 ≈ 0.23% C).
Group B steels have a guaranteed chemical composition. These steels are subjected to hot processing (forging, welding, heat treatment, TMT hardening, etc.). In this case, the mechanical properties are not preserved, and the chemical composition is important for determining the processing mode. They are marked: BSt. 1… BSt.6.
Steels of group B have guaranteed mechanical properties and chemical composition and are used like steel of group B. In grades of this steel, the letter B is placed first: VSt.1…VSt.5. Carbon steel of ordinary quality is cheap, its smelting accounts for about 80% of the total production of carbon steels.
From steels St.1, St.2, St. 3 groups A make fasteners, beams, etc., from St. 1, St. 2, St. 3 of groups B, C - cemented products, lightly loaded shafts, machine parts, St. 4 - used in shipbuilding, St. 5 , Art. 6 – used for the manufacture of medium-loaded parts (shafts, springs, springs, fasteners)
Carbon high-quality structural steels are marked with two-digit numbers indicating the average carbon content in hundredths of a percent and letters indicating the degree of deoxidation of the steel: steel 08, steel 10kp, steel 20, etc. With a steel content of 0.7-1% Mn in the grade steel, the letter G is added: 15G, 30G, 65G, etc. High-quality steels are supplied according to their chemical composition and mechanical properties.
Low-carbon structural steels are low-strength, highly plastic steels used for the manufacture of lightly loaded and case-hardened wear parts: gears, shafts, bushings, gaskets, etc.
Medium carbon steels are stronger and less ductile. They are used to make: spindles, rods, connecting rods.
High-carbon steels are strong with elastic properties and wear-resistant. The most critical parts are made from them - springs, leaf springs, etc.
Carbon tool quality steels are marked with the letter “U” and a number indicating the carbon content in tenths of a percent: U7, U8 ... U13. In high-quality steels, the letter A is placed at the end of the grade - U7A.
Alloy steels.
Alloyed steel is steel that contains alloying elements specially introduced into it in order to change its structure and properties. Alloying elements can form solid solutions with iron - alloyed ferrite and alloyed austenite, and a chemical compound - alloyed cementite or special carbides.
Alloy steels are classified:
- according to equilibrium structure
: hypoeutectoid steels (with excess ferrite), eutectoid (pearlitic structure) and hypereutectoid (with excess carbide) - these steels make up the pearlitic class, ledeburite, austenitic, ferritic;
- by composition
: nickel, chromium, chromium-nickel, etc.;
- by appointment
: structural, instrumental
,
with special properties; - by the number of alloying elements
: low-alloy steels up to 5%, medium-alloyed steels - 5...10%, high-alloyed steels - more than 10% of alloying elements;
- by quality
: high-quality, high-quality, especially high-quality;
Alloy steels are marked with letters and numbers indicating the approximate chemical composition of the steel.
In structural steels, the first two digits in the grade indicate the average carbon content in hundredths of a percent. The following shows the content of alloying elements. Each element is designated by its own letter: X - chromium, N - nickel, T - titanium, D - copper, G - manganese, C - silicon, A - nitrogen, K - cobalt, P - boron, F - vanadium, M - molybdenum, B – tungsten, Yu – aluminum.
The numbers after the letter indicate the approximate percentage of this element. The letter A at the end means that the steel is high quality, Ш - especially high quality.
In tool steels, the carbon content is indicated in one number and taken in tenths; if this figure is missing, then the carbon content is more than one percent, alloying elements and their quantity are designated as usual: 9ХС, ХВГ, ХВ5. For example, 9ХС steel contains 0.9% C and approximately one percent each of chromium and silicon.
For some groups of steels, different markings are used.
3.4. Classification and marking of cast iron
Iron-carbon alloys containing more than 2.14% C are called cast iron. In mechanical engineering practice, in most cases, cast iron containing 2.5...4.0% C is used.
Cast irons are classified according to their purpose, degree of graphitization or structure, form of graphite, microstructure of the metal base, and chemical composition.
According to their purpose, the groups are divided into conversion (for processing into steel) and foundry (for the production of castings).
According to the structure, cast iron is divided into white, gray and half-cast, depending on the form of separation C.
White
called cast iron, in which, under normal conditions, all carbon is in a bound state, mainly in the form of cementite. When broken, this cast iron has a white color and a characteristic metallic sheen. The presence of a large amount of high-hard cementite causes high brittleness and poor cutting performance. White cast iron is mainly processed into steel or transformed by heat treatment into malleable cast iron, sometimes used as a very wear-resistant material.
Gray
called cast iron, in which all or most of the carbon is in the form of graphite, and in a bound state (in the form of cementite) carbon contains no more than 0.8%. At the break it is gray in color.
In half
In cast iron, part of the carbon is in the form of graphite, but at least 2% C is present in the form of cementite.
According to the form of graphite, cast iron is divided into gray
– with lamellar graphite of varying degrees of vorticity and thickness of the plates;
malleable
- with flake-like graphite inclusions;
high-strength
- with spherical graphite inclusions.
Based on the structure of the metal base, cast irons are divided into ferritic
,
ferritic-pearlitic
and
pearlitic
.
According to the chemical composition, cast irons are divided into unalloyed, low-, medium- and high-alloyed, containing, respectively, 3...3.5%, 7...10% and more than 10% of alloying elements.
In addition to carbon, industrial cast iron necessarily contains silicon, manganese, sulfur and phosphorus.
Silicon promotes the graphitization of cast iron and is specially added; its content in cast iron ranges from 0.5% to 4.5%.
Manganese prevents graphitization and promotes the formation of Fe3C in the structure; the Mn content in cast iron is from 0.4 to 1.3%.
Sulfur is an undesirable element; it reduces fluidity and whitens cast iron. The S content is allowed no more than 0.08...0.12%.
Phosphorus is a useful admixture that improves fluidity, increases the hardness and wear resistance of cast iron. P content – 0.3…0.8%.
In addition to carbon and silicon, the structure of cast iron is significantly affected by the cooling rate of the castings. Rapid cooling produces white cast iron, and slow cooling produces gray cast iron. Gray cast iron is most widely used.
Gray cast iron
contains up to 3.8% C, while no more than 0.8% C is in the form of cementite, and the rest of the carbon is in the form of graphite plates - flakes.
The metal base of gray cast iron can be Φ, Φ+Π, Π, while the structure does not affect the ductility of gray cast iron (still low), but does affect its hardness and strength.
Graphite has low mechanical strength, and the places where it occurs can be considered as internal cuts, cracks, and discontinuities. The more graphite and the larger the inclusions, the lower the mechanical characteristics. To grind graphite inclusions, liquid cast iron is modified by adding silicocalcium, aluminum and ferrosilicon.
Gray cast iron is widely used in mechanical engineering. It is a cheap metal with good casting properties. It is easy to process with cutting tools and has good anti-friction and damping properties.
Rice. 3.4. Influence of the metal base and the shape of inclusions
graphite on the properties of cast iron
Gray cast irons are marked with the letters SCH (gray cast iron) and numbers indicating tensile strength (tensile strength σв). For example: SCh12, SCh18, SCh21, SCh36, SCh40, etc.
Cast irons SCh12 - SCh18 are used for the manufacture of non-critical parts: covers, bearing housings, foundation plates, etc.
Cast iron, starting with SCh21, is used for the manufacture of powerful machine tool beds, critical parts, gears, etc.
When castings are rapidly cooled, graphitization can occur only in the middle of the casting, and the surface acquires the structure of white or half cast iron. Such gray cast iron castings are called bleached; they have good wear resistance; rolls and balls for mills, brake pads, etc. are made from them.
Ductile iron
contains about 3.0...3.6% C. It is obtained by adding magnesium (0.03...0.07%) or other alkali or alkaline earth metals to liquid cast iron. In this case, the released graphite acquires a spherical shape, such graphite weakens the metal base less, and the mechanical properties of cast iron improve - its ductility increases and its hardness increases. The metal base of high-strength cast iron can also be different: F, F+P, P.
High-strength cast irons are marked with the letters VC and numbers indicating tensile strength in kgf/mm2 and elongation in%, for example: VC38-47, VC40-10, VC50-2.5, VC60-2, etc.
High-strength cast iron is used to make rolling mill equipment, press-forging equipment, internal combustion engine housings, large shafts and other critical parts.
Malleable iron
contains: 2.2...3.0% C, 0.7...1.5% Si, 0.2...0.6% Mn, less than 0.2% P and less than 0.1% S. The term “malleable cast iron” is conventional and reflects the increased tensile ductility of this cast iron compared to other types.
Malleable iron is produced by annealing white iron castings, causing the cementite to disintegrate and the graphite to flake out.
When annealing, white cast iron products are heated above temperature A1 (950...1000°C), held for about 15 hours, slowly cooled for 30 hours in zone A1 (eutectoid transformation temperature) from 760°C to 720°C and then cooled to room temperature temperature.
Rice. 5.4. Annealing schemes for white cast iron for ferritic (1) and pearlitic (2) malleable cast iron
At t = 950°C, cementite decomposes Fe3C → 3Fe + G, and then at t = 760...720°C, austenite decomposes A → F + G.
As a result of all transformations, the structure of malleable cast iron will consist of grains F and evenly distributed flakes G. Since such cast iron contains quite a lot of graphite, the fracture turns out dark and is called black-hearted
(F + G) – malleable ferritic cast iron.
If in the region of the eutectoid transformation the cooling rate is higher, then the cast iron may have a pearlite and graphite structure, i.e. P + G, such cast iron is called malleable pearlitic cast iron, or light-hearted
.
Malleable cast iron is marked with the letters KCH and numbers of tensile strength and relative elongation, for example: KCH30-6, KCH50-4, KCH60-3, etc.
Malleable ferritic cast iron is used to make both products operating under high static and dynamic loads (gearbox housings, hubs, edges) and less critical parts (clamps, nuts, coupling flanges).
Malleable pearlitic cast iron is used to make driveshaft forks, conveyor links and frames, bushings, and brake pads.
Malleable cast iron is used for small cross-section parts operating under shock and vibration loads.
Alloyed
and
special
cast irons are obtained by introducing additives of alloying elements. Cr, Ti, V, etc. are used as additives. Special cast irons differ in their silicon and manganese content.
Cast irons are marked differently, for example, antifriction: AChS-1, AChK-1, AChV-1 or AChS-2, AChK-2, etc., silicon (14-18% Si): C-15, C- 17, heat-resistant: ZhChKh-20 (20% Cr), ZhChKh-22, etc.
Polymorphic transformations
More details about each phase are given below in the article. In short, the main transformations occur at special temperatures.
The state of iron is designated as α-ferrum (at a temperature less than 911 ° C). The crystal lattice is a volumetric face-centered cube. Or OCC. The distance between the atoms of such a lattice is quite high.
Iron acquires the gamma modification, that is, it is designated as γ-ferrum (911-1392° C). The crystal lattice is a face-centered cube (FCC). In this lattice the distance between atoms is lower than in bcc.
When α-ferrum transforms into γ-ferrum, the volume of the substance becomes smaller. The reason for this is the crystal lattice - its appearance. Because the fcc lattice has a more ordered state of atoms than the bcc lattice.
If the transition is carried out in the opposite direction - from γ-ferrum to α-ferrum, then the volume of the alloy increases.
When the temperature reaches 1392° C (but less than the melting point of iron 1539° C), then α-ferrum turns into δ-ferrum, but this is not its new form, but only a variety. In addition, δ-ferrum is an unstable structure.
Physics 8th grade. Melting and crystallization
Physics 8th grade Notes Melting Melting and crystallization. Specific heat of fusion.
Problems on the topic Thermal phenomena
The transition of a substance from solid to liquid is called melting.
Melting of crystalline bodies occurs only at a certain temperature.
The temperature at which a substance melts is called the melting point of the substance.
To carry out the melting process, the solid must first be heated to its melting point.
If a body is heated to the melting temperature and the heater is removed (stop supplying heat to the body), then melting does not occur.
To melt a body, two conditions must be met: 1. heat the body to the melting temperature 2. continue the transfer of heat
Melting point is an important thermal characteristic of a substance. Different substances have different melting points.
Melting metal
The transition of a substance from a liquid to a solid state is called solidification or crystallization.
For crystallization of a molten (liquid) body to begin, it must cool to a certain temperature.
The temperature at which a substance hardens (crystallizes) is called the solidification or crystallization temperature.
Graph of melting and solidification of crystalline solids.
Melting and solidification schedule
Experience shows that substances solidify at the same temperature at which they melt.
To carry out the solidification process, two conditions must be met: 1. cool the liquid to the solidification (melting) temperature 2. continue to remove heat until all the liquid has solidified.
A physical quantity showing how much heat must be imparted to a crystalline body weighing 1 kg in order to completely transform it into a liquid state at the melting point is called the specific heat of fusion.
The specific heat of fusion is denoted by the letter λ and measured in J/kg.
To calculate the amount of heat Q required to melt a crystalline body of mass m, taken at its melting point and normal atmospheric pressure,
you need to multiply the specific heat of fusion λ by the body mass:
where Q is the amount of heat, m is body mass.
The melting and crystallization temperatures for a given substance at constant external pressure are equal.
Crystallization
The amount of heat released during the crystallization of a substance, at a constant external pressure, is equal to the amount of heat received by this substance during melting.
Tasks
Evaporation and condensation
The abstract was compiled based on the theoretical material of the textbook “Physics 8th grade” by A.V. Peryshkin, “Physics 8th grade” A.V. Grachev.
Download abstract:
teoriya_8_plavleniekristallizacziya
Physics 8th grade. Quantity of heat. Specific heat. Fuel.
Physics 8th grade. Boiling. Condensation.
Physics 8th grade. Evaporation. Saturated steam. Air humidity.
Physics 8th grade. Thermal phenomena. Internal energy.
Properties of commercially pure iron
Magnetic properties of iron at different temperatures:
- less than 768° C – ferromagnetic;
- more than 768° C – paramagnetic.
And the temperature point of 768° C is called the magnetic transformation point, or the Curie point.
Properties of technically pure iron:
- hardness – 80 HB;
- temporary resistance - 250 MPa;
- yield strength – 120 MPa;
- relative elongation 50%;
- relative narrowing – 80%;
- high modulus of elasticity.
Iron carbide
Graphical view of the constituent part of the iron-carbon diagram: Fe3C. The substance is called iron carbide, or cementite. It is characterized by:
- Carbon content 6.67%.
- Specific gravity - 7.82%.
- The crystal lattice has a rhombic shape, consisting of octahedra.
- Melting occurs at a temperature of ≈1260° C.
- Low ferromagnetic properties at low temperatures.
- Hardness – 800 HB.
- Plasticity is practically zero.
- Iron carbide forms substitutional solid solutions in which carbon atoms are replaced by non-metal atoms (nitrogen), and iron atoms by metals (chromium, tungsten, manganese). This solid composition is called alloyed.
As noted above, cementite is an unstable phase, and graphite is stable. Since the first substance is an unstable compound, decomposing under certain temperature conditions.
The iron-carbon diagram has the following states:
- liquid phase;
- ferrite;
- austenite;
- cementite;
- graphite;
- perlite;
- ledeburite.
Let's look at each of them in detail.
Ferrite
It is a solid solution of carbon interstitial in α-ferrum. Small amounts of impurities may also be included. But ferrite has almost the same qualities as pure iron. If you examine the structure under a microscope, you can see light-colored polyhedral grains.
Happens:
- low temperature (at a temperature of 727° C, carbon solubility is 0.02%);
- high-temperature (at 1499 ° C, carbon solubility is 0.1%), or it is called δ-ferrum.
Ferrite properties:
- hardness - 80-120 HB;
- temporary resistance - 300 MPa;
- relative elongation - 50%;
- has good magnetic properties (up to a temperature of 768° C).
Structure and properties
As the temperature increases, austenitic steels turn into a liquid solution with a certain percentage of iron and carbon. If the temperature of the solution exceeds the so-called liquidus line (about 1700 °C), the resulting melt becomes statically unstable. Its condition is assessed by two components: phase and structural.
For the first component, the main indicator is the state phase of the resulting mixture. It determines the condition of the metal according to the following indicators:
- solution of carbon in iron;
- number of different formations (ferrite itself, including high-temperature ferrite, austenite, cementite).
The structural component of a sample is defined as a homogeneous or quasi-homogeneous form. The general structure of the resulting ferrite is equiaxed crystals. In three-dimensional space, the ferrite phase lattice represents a body-centered cube. These crystals determine the hardness of ferrite and the ability of carbon to dissolve in it. Experience shows that at a temperature of 727 degrees only 0.02% of carbon dissolves in ferrite.
In addition, the main properties of ferrite include:
- has strong ferromagnetic properties (up to a temperature of 770 ° C - Curie point);
- is a heat-conducting element;
- good conductor of electric current;
- has increased plasticity.
The main disadvantages include low strength and insufficient hardness. The latter indicator depends on the size of the grain formed and is in the range from 65 to 130 HB.
Depending on the stage of ongoing transformations, the ferrite phase is in the following states:
- as the basis of the crystal lattice of the resulting alloy;
- second or excess state (located along the boundaries of the so-called pearlite formations);
- ferrite-graphite eutectoid element.
Each condition requires precise definition and identification of emerging transformations. The characteristics of the final product largely depend on them. The complete absence of ferrite formation or its insignificant content manifests itself with the formation of hot cracks. An overestimated content of this indicator reduces ductility, impact strength and anti-corrosion resistance.
Austenite
This is a solid solution of interstitial carbon in γ-ferrum. There may also be small amounts of impurities. In a crystal lattice, carbon is located in the center of the fcc cell. When examining the structure of austenite under a microscope, it is visible as light polyhedral grains with twins.
Has the following characteristics:
- The solubility of carbon in γ-ferrum is 2.14% (at a temperature of 1147 ° C).
- Austenite hardness 180 HB;
- Elongation - 40-50%;
- Good paramagnetic qualities.
Cementite and its forms
Present in the following phases: Ts1, Ts2, Ts3 (primary, secondary and tertiary cementite).
As for the physicochemical indicators of these three states, they are approximately equal. Mechanical properties are affected by particle size, number and location.
The diagram also shows that:
- C1 is formed from a liquid state (under a microscope it is visible as large plates);
- Ts2 – from austenite (located around its grains in the form of a network);
- C3 – made of ferrite (located at the boundaries of ferrite grains in the form of small particles).
Description
The carbon concentration in cementite—6.67% by weight—is the limit for iron-carbon alloys. Cementite is a metastable phase; the formation of a stable phase—graphite—is difficult in many cases. Cementite has an orthorhombic crystal lattice, is very hard and brittle, and weakly magnetic up to 210 °C.
Depending on the conditions of crystallization and subsequent processing, cementite can have different shapes - equiaxed grains, a network along grain boundaries, plates, as well as a Widmanstätt structure.
Cementite in different quantities, depending on the concentration, is present in iron-carbon alloys even at low carbon contents. Formed during the process of crystallization from molten cast iron. In steels it is released when austenite is cooled or martensite is heated. Cementite is a phase and structural component of iron-carbon alloys, an integral part of ledeburite, perlite, sorbite and troostite. Cementite is a representative of the so-called interstitial phases, compounds of transition metals with light metalloids. In the interstitial phases, the proportion of both covalent and metallic bonds is high.
Brinell hardness is more than 800 kg/mm2. Primary cementite crystallizes from a liquid alloy Secondary cementite - from austenite Tertiary cementite - from ferrite
This is interesting: Cementing steel at home with graphite and other methods - we analyze it thoroughly
Cast iron
Alloys on the iron-carbon diagram that contain more than 2.14% carbon are called cast irons. They are highly fragile. The cross section of such cast iron has a light tone, and therefore it is called white cast iron.
On the diagram this is point C, called the eutectic, with a corresponding carbon content of 4.3%. Crystallization produces a mixture of austenite and cementite, collectively called ledeburite. The phase composition is constant.
When the carbon concentration is less than 4.3% (hypoeutectic cast iron), austenite is released from the solution during crystallization. Next, C2 is isolated from it. And at 727° C, austenite turns into pearlite. The structural state of such cast iron is as follows: large areas of dark-colored pearlite.
In hypereutectic white cast iron (carbon more than 4.3%), upon cooling, structuring occurs with the formation of C1 crystals. Further transformations are carried out in the solid state. The structure is ledeburite, which is the background for fields of dark-toned pearlite. And large layers are Ts1.
Phases in the iron-carbon system
Phases are physically homogeneous states of the alloy. The phase has a precise chemical composition - a specific arrangement and connection between atoms. This atomic structure gives different properties to different phases.
Some special alloys can exist in several phases, which is achieved by heating the metal to certain temperatures and using different heat treatment procedures.
Liquid
Exists at temperatures that exceed 1457 oC. Phase transformation at this temperature means complete melting, so the liquid phase is always indicated by the line L on the diagram.
Ferrite
Present in the diagram in three different phases:
- The delta ferrite (δ-Fe) phase is a solid solution of C in δ-Fe (bcc) in the high temperature region of the diagram. The solution is stable above 1400 °C and melts above 1539 °C. Structurally similar to α-ferrite;
- The gamma ferrite (γ-Fe) phase is a solid solution of C in γ-Fe, which is called austenite. Unstable below 910°C, becoming δ-ferrite at 1395°C. Maximum carbon solubility is about 2.1% at 1147°C. Austenite is soft and ductile and does not have magnetic properties;
- The alpha ferrite phase (α-Fe) is a solid solution of C in α-Fe with a bcc lattice. It is considered the most stable form of iron at room temperature. The maximum solubility of carbon is about 0.02% at 727 °C. Softer than austenite, magnetic.
Austenite in steels
Austenite is always present in stainless steels, which contain from 16 to 26 percent chromium and up to 35 percent nickel. Austenitic steels, in addition to high corrosion resistance, are not hardened by heat treatment and are non-magnetic.
The forms of existence of cementite are important for determining the corrosion resistance of steels. Cementite (Fe3C) has been shown to increase corrosion rates, with this effect being more pronounced when it forms a coherent network on the surface. In normalized steels, cementite forms a coherent network, but in tempered martensite it does not. Therefore, cementite affects the corrosion rate of normalized but not hardened steel.
In general, the corrosion rate of carbon steel decreases with increasing chromium content due to the formation of protective chromium oxide. However, when chromium combines with carbon to form chromium carbide, the positive effect of chromium is lost.