Let's talk about solids. Solids can be divided into two large groups: amorphous and crystalline. We will separate them according to the principle of whether there is order or not.
In amorphous substances, molecules are arranged randomly. There are no patterns in their spatial arrangement. Essentially, amorphous substances are very viscous liquids, so viscous that they are solid.
Hence the name: “a-” – negative particle, “morphe” – form. Amorphous substances include: glass, resins, wax, paraffin, soap.
The lack of order in the arrangement of particles determines the physical properties of amorphous bodies: they do not have fixed melting points. As they heat up, their viscosity gradually decreases, and they also gradually turn into a liquid state.
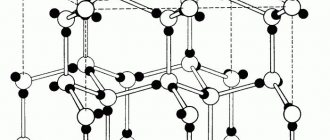
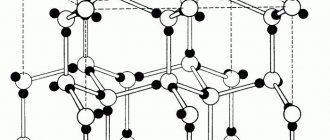
In contrast to amorphous substances, there are crystalline substances. The particles of a crystalline substance are spatially ordered. This regular structure of the spatial arrangement of particles in a crystalline substance is called a crystal lattice.
Unlike amorphous solids, crystalline substances have fixed melting points.
Depending on what particles are in the lattice nodes and what bonds hold them together, they are distinguished into molecular, atomic, ionic and metal lattices.
Why is it fundamentally important to know what kind of crystal lattice a substance has? What does it define? All. Structure determines both the chemical and physical properties of a substance.
The simplest example: DNA. In all organisms on earth, it is built from the same set of structural components: four types of nucleotides. And what a variety of life. This is all determined by structure: the order in which these nucleotides are arranged.
Definition
As we know, all material substances can exist in three basic states: liquid, solid, and gaseous. True, there is also a state of plasma, which scientists consider no less than the fourth state of matter, but our article is not about plasma. The solid state of a substance is therefore solid because it has a special crystalline structure, the particles of which are in a certain and clearly defined order, thus creating a crystal lattice. The structure of the crystal lattice consists of repeating identical elementary cells: atoms, molecules, ions, and other elementary particles connected by various nodes.
Seven types of crystal lattices
There are seven different crystal systems. They were discovered in 1781 by Father Rene Just Howie. He accidentally noticed that some stones had an ideal shape. After many years of research, he developed his theory about the structure of crystals. In 1848, Auguste Bravais shows that there can only be seven types of elementary crystalline networks.
Systems characterize the different geometric shapes that a crystalline network can have.
Each of these systems is defined by its axes: three dimensional parameters (the length of the axes) and three angular parameters (the angles formed by the two axes). Conventionally, we call abc the lengths of the axes and α β and γ the angles formed by the axes. They are placed in space as follows:
Each cell representing a system also has a certain number of symmetries. These symmetries are of three types:
- central (marked C): the point is the center of symmetry of the mesh;
- plane (marked P): the plane is the plane of symmetry of the mesh;
- axial (O): rotation by a certain angle around the axis of symmetry returns the mesh to a position identical to the original one.
These symmetries have four orders:
- binary (labeled L2): 180° rotation (π rad)
- ternary (labeled L3): 120° rotation (2π/3 rad)
- quaternary (marked L 4): Rotation by 90° (π/2 rad.)
- hexagonal (marked L 6): 60° rotation (π/3 rad.)
Cubic (or isometric) lattice
| a = b = c: the three axes have the same length α = β = γ= 90°: the three angles are equal and straight Symmetries: C, 3 L 4, 4 L 3, 6 L 2, 9 P The basic element is the cube. |
Quadratic (or tetragonal) lattice
| a = b ≠ c: two axes have the same length, but the third axis is different. α = β = γ= 90°: three angles are equal and straight Symmetries: C, L 4, 4L2, 5 P The main element is a right-handed prism with a square base. |
Orthorhombic crystal lattice
| a ≠ b ≠ c: the three axes have different lengths α = β = γ= 90 °: the three angles are equal and straight Symmetries: C, 3 L 2, 3 P The main element is a cuboid. |
Monoclinic lattice
| a≠b≠c: The three axes are of unequal length. β = γ= 90 °≠α: two angles are equal and right. Symmetries: C, L 2, P The main element is an inclined prism, at the base of which is a rhombus. |
Triclinic lattice
| a≠b≠c: The three axes are of unequal length. α≠β ≠ γ≠ 90°: the three angles are different. Symmetries: C, L 2, P The main element is a parallelepiped with a rhombus base. |
Rhombohedral lattice
| a = b = c: three axes have the same length α = β = γ≠ 90 °: three angles are equal and straight Symmetries: C, L 3, 3 L 2, P The main element is a parallelepiped, all planes of which are rhombuses. |
Types of gratings
Depending on the particles of the crystal lattice, there are fourteen types of it, here are the most popular of them:
Metal crystal lattice.
Next, we will describe in more detail all types of crystal lattice.
Ionic type
Oppositely charged ions are located at nodes that create an electromagnetic field that characterizes the physical properties of a substance. These will include: electrical conductivity, refractoriness, density and hardness. Table salt and potassium nitrate are characterized by the presence of an ionic crystal lattice.
Don't miss: the mechanism of metal bond formation, specific examples.
Atomic lattice
Substances with an atomic crystal lattice, as a rule, have strong covalent bonds in their nodes consisting of atoms themselves. A covalent bond occurs when two identical atoms share fraternal electrons with each other, thus forming a common pair of electrons for neighboring atoms. Because of this, covalent bonds bind atoms tightly and evenly in a strict order - perhaps this is the most characteristic feature of the structure of the atomic crystal lattice. Chemical elements with similar bonds can boast of their hardness and high melting point. Chemical elements such as diamond, silicon, germanium, and boron have an atomic crystal lattice.
Molecular crystal lattice
The nodes of this structure contain molecules that are tightly packed together. Such substances are characterized by covalent polar and nonpolar bonds. It is interesting that, regardless of the covalent bond, there is a very weak attraction between the particles (due to weak van der Waals forces). That is why such substances are very fragile, have low boiling and melting points, and are also volatile. These substances include: water, organic substances (sugar, naphthalene), carbon monoxide (IV), hydrogen sulfide, noble gases, two- (hydrogen, oxygen, chlorine, nitrogen, iodine), three- (ozone), four- (phosphorus ), eight-atomic (sulfur) substances, and so on.
One of the distinctive features is that the structural and spatial model is preserved in all phases (both solid, liquid and gaseous).
Metal grate
The type of bond of a metal crystal lattice is more flexible and ductile than the ionic one, although in appearance they are very similar. Its distinctive feature is the presence of positively charged cations (metal ions) at lattice sites. Between the nodes live electrons that participate in the creation of the electric field; these electrons are also called electric gas. The presence of such a structure of a metal crystal lattice explains its properties: mechanical strength, heat and electrical conductivity, fusibility.
Covalent crystals
The nodes of such crystal lattices contain individual atoms or molecules connected to each other by covalent bonds.
A covalent bond is a chemical bond that involves sharing pairs of electrons between atoms. This separation results in a stable balance of attractive and repulsive forces between these atoms. Covalent solids are a class of compounds with extended lattices in which each atom or molecule is covalently bonded to its nearest neighbors. This means that the entire crystal is essentially one giant molecule. The extremely strong binding forces that bind all adjacent atoms together explain the extreme hardness of these solids. They cannot be broken or abraded without breaking a large number of covalent chemical bonds. Likewise, a covalent solid cannot "melt" in the usual sense, since the entire crystal is one giant molecule. When heated to very high temperatures, these solids typically decompose into their elements.
Another property of covalent solids is poor electrical conductivity , since there are no delocalized electrons in such substances. In the case of melting, unlike ionic compounds, such substances are also unable to conduct electricity, since their macromolecules consist of uncharged atoms, not ions.
Now, knowing the type of chemical bond in a substance, it is possible to characterize not only its quantitative and qualitative composition, but also its physical properties.
Video
And finally, a detailed video explanation about the properties of crystal lattices.
Author: Pavel Chaika, editor-in-chief of Poznavaika magazine
When writing the article, I tried to make it as interesting, useful and high-quality as possible. I would be grateful for any feedback and constructive criticism in the form of comments on the article. You can also write your wish/question/suggestion to my email [email protected] or Facebook, with respect, the author.
Author page
This article is available in English - Crystal Lattice in Chemistry.
Various substances
- Diamond. The mineral is of high value and, after cutting, is used in jewelry. So what is the secret of the popularity of this stone? Carbon atoms form the basis of the entire lattice. There is a strong covalent bond between the atoms of the mineral. The crystal lattice of diamond is characterized by a dense content of atoms in the form of a cube. In other words, carbon atoms are considered nodes, and the peculiar faces of the cube are strong covalent bonds. This mineral is considered the most durable on the planet, and it is unknown how many of these unique cubes include a solid diamond.
- Graphite. Carbon can also be in another crystalline modification. The atomic lattice of this element includes only carbon atoms and has a layered structure. In graphite, each atom is bonded by three carbon atoms. Because of this, it has a metallic luster and high thermal conductivity.
- The crystal lattice of iodine is of a molecular type. Atoms of molecules are connected by covalent bonds, but the molecules of a chemical element have weak attractive forces. This characterizes iodine in that it has low hardness and a low melting point.
- Sodium. Representative of a metal crystal lattice. Electrons move between cations located at lattice sites. By joining cations, they neutralize their charge, in turn, neutral atoms release some electrons, transforming into cations. This type of crystal lattice gives the metal plasticity, electrical and thermal conductivity.
- Dry ice. Or carbon monoxide in solidified form. It has a molecular crystal lattice in the shape of a cube. Molecules are held together by weak bonds. infusion, read our article.
This is interesting: how to determine valency using the periodic table?
Energy level
The differences between nonmetals and metals are primarily due to the structure of their atoms. Let's start with the number of electrons in the outer energy level. For metal atoms it varies from one to three. As a rule, they have a large radius, so metal atoms easily give up their outer electrons, since they have strong reducing properties.
Nonmetals have a larger number of electrons in the outer level. This explains their oxidative activity. Nonmetals add missing electrons, completely filling the energy level. The strongest oxidizing properties are exhibited by non-metals of the second and third periods of groups VI-VII.
A filled energy level contains 8 electrons. The halogens with valence I have the greatest oxidizing ability. Fluorine is the leader among them, since this element has no free orbitals.
What are semimetals
In the periodic table, between metals and non-metals, there are a number of chemical elements that occupy an intermediate position. They are called semimetals. Semimetal atoms are linked by covalent chemical bonds.
These substances combine the characteristics of metals and non-metals. For example, antimony is a silvery-white crystalline substance that reacts with acids to form salts—typical metallic properties. On the other hand, antimony is a very fragile substance that cannot be forged, and it can even be crushed by hand.
So, typical non-metals and metals have opposite properties, but this division is quite arbitrary, since a number of substances combine both characteristics.