The influence of carbon on the properties of steels
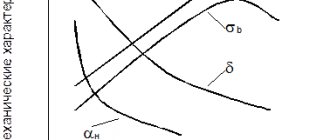
Carbon is the main strengthening element in all steels except austenitic stainless steels and some other high-alloy steels. The strengthening effect of carbon consists of solid solution strengthening and strengthening due to dispersed precipitation of carbides. With increasing carbon content in steel, its strength increases, but ductility and weldability decreases.
Carbon has a moderate tendency to macrosegregate during crystallization. Macrosegregation of carbon is usually more significant than that of all other alloying elements. Carbon has a strong tendency to segregate at defects in steels such as grain boundaries and dislocations. Carbide-forming elements can react with carbon to form “alloyed” carbides.
Manganese steel grades
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Products Steel castings Drawings (Steel)
- Drawings (Manganese steel)
- Manganese steel
- Heat resistant steel
- Casting steel
- Structural alloy steel
- Alloy steel
- Carbon steel
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Steel casting Drawings (Steel)
Drawings (Manganese steel)
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
The influence of manganese on the properties of steels
Manganese is present in almost all steels in amounts of 0.30% or more. Manganese is used to remove oxygen and sulfur from steel. It has less tendency to segregate than any other alloying element. Manganese has a beneficial effect on surface quality over the entire carbon content range, with the exception of very low carbon steels, and also reduces the risk of red brittleness. Manganese has a beneficial effect on the ductility and weldability of steels.
Manganese does not form its own carbide, but only dissolves in cementite and forms alloyed cementite in steels. Manganese promotes the formation of austenite and therefore expands the austenite region of the phase diagram. High manganese content (more than 2%) leads to an increased tendency to cracking and warping during hardening. The presence of manganese in steels encourages impurities such as phosphorus, tin, antimony and arsenic to segregate to the grain boundaries, causing temper brittleness.
Manganese steel - grade
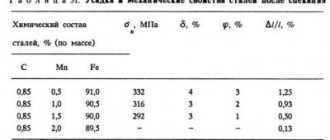
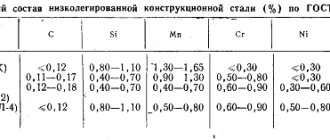
Low-carbon manganese steels of grades 10G2A and 12G2A have high ductility and good weldability. They are used for the manufacture of stamp-welded parts. [1]
When processing manganese steel grade G12 (manganese content 12–94%), it was found that the most suitable for such processing is a cutter made of hard alloy grade T15K6, which has sufficient durability at a cutting speed of 13–6 m/min. The T5KYu alloy provides satisfactory durability (50 min. However, the T15K6 alloy is relatively brittle and does not work well under impact loads. [2]
Crankshaft 3 is made of manganese steel grade 50G and rests on five main bearings. The surfaces of the shaft journals are hardened by high frequency currents. The diameter of the main neck is 88-9 mm, the diameter of the crank is 70 mm. To balance the centrifugal forces, counterweights are installed on the first and fourth cranks of the shaft. [3]
Steel with high wear resistance is manganese steel grade G13, containing 1 0 - 1 3% C; 11 0 - 14 0% MP. It belongs to the austenitic class. [4]
For welding main pipelines, wire made of carbon steel grades SV-08 and SV-08A and manganese steel grades SV-08g-A is used. The letter A in the wire grade means that the wire contains significantly less harmful impurities - sulfur and phosphorus, therefore such wire is used for more demanding work. [5]
The regenerator consists of housing 1, the lower part of which (up to the flange connection) is made of chromium-nickel steel grade X18N9T, and the upper part is made of manganese steel grade 09G2DT / m; 2 coils made of copper or steel tubes, and 3 stone nozzles with a granule size of 4 - 10 mm. The coils rest on the bottom of the housing. The coil collectors are routed through seals 4 and 5, located in the bottom and cover. Air is introduced into the regenerator and the return flow is output through a perforated cone 6, covered with a stainless steel mesh 7, and the air is removed and the return flow is introduced through an annular perforated manifold 8, also covered with a stainless steel mesh. [6]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0.1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade PZ, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [7]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0 1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade G13, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [8]
In bins designed to store solid lump materials, the inner surface of the inclined walls is lined to protect the walls from abrasion and dents due to impact. The type of lining depends on the abrasive properties of the bulk material. Thus, bunkers for ore and scrap are lined with sheet manganese steel grade ZOG2 with a thickness of 6 - 10 mm. Sometimes wooden lining is used. [10]
The influence of silicon on the properties of steels
Silicon is one of the main deoxidizing agents used in steel smelting. Therefore, the silicon content determines the type of steel produced. Mild carbon steels can contain silicon up to a maximum of 0.60%. Semi-quiet steels may contain moderate amounts of silicon, for example 0.10%.
Silicon is completely dissolved in ferrite at a silicon content of up to 0.30%. It increases the strength of ferrite without almost reducing its ductility. When the silicon content is above 0.40% in general purpose carbon steel, a significant decrease in ductility occurs.
In combination with manganese or molybdenum, silicon provides higher hardenability of steel. The addition of silicon to chromium-nickel austenitic steels increases their resistance to stress corrosion. In thermally hardenable steels, silicon is an important alloying element; it increases the ability of steels to be thermally hardened and their wear resistance, increases the elastic limit and yield strength. Silicon does not form carbides and does not contain cementite or other carbides. It dissolves in martensite and slows down the decomposition of alloyed martensite up to 300 °C.
Manganese alloying
Alloying chromium steels with small additions of aluminum significantly increases their heat resistance. Thus, the addition of 2% Al to steel with 6% Cr almost completely prevents the oxidation of steel at 800° C (Fig. 167). By adding 5% aluminum to steel containing 30% Cr, it is possible to obtain an alloy suitable for use at temperatures up to 1300°C, but which is brittle, since aluminum deteriorates the mechanical properties of the alloy. The best corrosion resistance in an atmosphere of hydrogen sulfide is possessed by high-chromium steels of 25 -27% Sgn3-5% L1.Alloying chromium steels with titanium at a ratio of atomic amounts of Cr/Ti -7...10 and Cr/C = 0.4...0.7 leads to the complete disappearance of cementite carbide during annealing and the appearance of cubic carbide.
Additional alloying of chromium steels with zirconium affects the structure similarly to titanium. Chromium is dissolved in the resulting granular ZrC carbides, as in titanium carbides.
Alloying chromium steels with zirconium compared to titanium creates the possibility of increasing the abrasive wear resistance of steels both in cast and heat-treated states with a significantly lower chromium content (3.4%).
c) adding alloying elements that promote the cathodic process and thereby self-passivation of the metal (for example, alloying chromium and chromium-nickel steels with a small amount of platinum or palladium in order to increase the resistance of these alloys to atmospheric corrosion and gray cast iron to the action of nitric acid by alloying with copper, etc. .);
With an increase in the concentration of chromium in steel, the resistance of the latter in water at high temperatures increases. Thus, at a temperature of 160° C in water containing 6.004 mg/l of oxygen, for steel alloyed with 5% chromium, the corrosion rate decreases by 3.5 times [111,148]. An increase in chromium concentration to 12% under the same conditions affects the rate of the corrosion process unnoticed. In cases where the material must not only be resistant to corrosion, but also resistant to erosion, the advantage of chromium steels is even more obvious. If, for example, in distilled water at elevated temperature and pressure we take the durability of pump parts made of carbon steel as 1, then the durability of chromium steels with a chromium concentration of 5–13% is 100–105 [111,149]. In the vapor phase, according to J-Knox [111,150], if steel is alloyed with 5% chromium, the corrosion rate almost does not decrease. It decreases only if the chromium concentration in the steel is 9%. Chromium steels are more resistant than carbon steels in solutions containing sodium chloride. Thus, steel alloyed with 3.7% chromium and 1.3% aluminum has corrosion resistance in seawater five times higher than that of carbon steel [111,151]. J. B. Godshall [111,149] notes that pump parts made of steel alloyed with 5% chromium and 0.5% molybdenum were in satisfactory condition after 50,000 hours of operation. Parts made of carbon steel failed due to corrosion damage after only 500 hours. As mentioned above, in solutions containing chlorine ions, chromium steels are susceptible to local corrosion. Alloying chromium steels with a small amount of copper and molybdenum did not change the essence of the matter [111,152].
In connection with the increase in operating temperatures of steam power plants and power compressors of gas turbine plants, complex alloyed 12% chromium stainless and heat-resistant steels have been created and become widespread (1Х11МФ, 1Х12ВНМФ, 1Х12В2МФ, 2Х12ВМБФР, 1Х12В4МФ, 13Х14Н2ВФР, 1Х12Н2ВМ F, -21Х15НЗМА, 1Х16Н4Б, etc. ). Additional alloying of chromium steels with molybdenum, tungsten, vanadium, niobium, and boron made it possible, along with maintaining stainless properties, high hardenability, ability to harden in air, and a low expansion coefficient, to provide higher heat-resistant properties (preservation of strength at 550-600 and up to 650 ° C for short service periods).
Alloying chromium steels with nickel significantly increases hardenability and leads to a significant decrease in critical points, °C: Ls, 680–700, Asya 820–840, Mn 160–180 (Table 1.2). This circumstance seriously complicates mitigating
c) adding alloying elements that promote the cathodic process and thereby self-passivation of the metal (for example, alloying chromium and chromium-nickel steels with a small amount of platinum or palladium in order to increase the resistance of these alloys to atmospheric corrosion and gray cast iron to the action of nitric acid by alloying with copper, etc. .);
From Fig. 51 shows the great influence of Mo on the change in current in the passive state. Alloying chromium alloys with 1% Mo at not very high chromium contents reduces the current value in the passive state Δpp by 10 times [124].
These coatings are weakly oxidizing, and therefore allow the weld metal to be alloyed with elements with a high affinity for oxygen. The presence of a large number of calcium compounds, which bind sulfur and phosphorus well and remove them into the slag, ensures high purity of the deposited metal, its increased plastic properties, and alloying with manganese and silicon provides high strength. Seams made with such electrodes are highly resistant to the formation of hot cracks and have the highest (compared to any other coatings) impact strength. The ac value is at least 13 kgf-m/cm2 and can reach 25 kgf-m/cm2.
The presence of manganese improves the corrosion and mechanical properties, although they are low for this alloy. Alloying with aluminum increases the strength properties due to the formation of a solid solution stronger than alloying with manganese, therefore, when adding 3-4% Al to the alloy, the strength is increased;; to 30 kgf/mm2. Naturally, an alloy with 3-4% Al is not yet capable of noticeable strengthening during heat treatment. The same can be said about the MA1 alloy, while the MA5 and MAYu alloys, containing 8-9% Al, have a strength after quenching (about 400°C) and aging (175°C) of the order of 40 kgf/mm2.
Austension steels get their name from the austenitic phase or y-phase, which exists in pure iron in the form of a stable structure in the temperature range from 910 to 1400 ° C. This phase has a face-centered cubic lattice, is non-magnetic and is easily deformed. It is the main or sole phase of austenitic stainless steels at room temperature and, depending on the composition, has a stable or metastable structure. The presence of nickel significantly contributes to the preservation of the austenite phase during quenching of industrial Cr-Fe-Ni alloys from high temperatures. An increase in nickel content is accompanied by an increase in austenite stability. Alloying with manganese, cobalt, carbon and nitrogen also contributes to the retention and stabilization of austenite during quenching. Austenitic stainless steels can be hardened by cold working, but not by heat treating. During cold working, austenite in metastable alloys (for example, 201, 202, 301, 302, 302B, 303, 330Se, 304, 304L, 316, 316L, 321, 347, 348; see Table 18.2) partially transforms into ferrite . For this reason, these steels are metastable. They are magnetic and have a body-centered cubic lattice. This transformation explains the significant degree of hardening during mechanical processing. At the same time, steels 305, 308, 309, 309S are weakly hardened during cold working, and even if they become magnetic, it is to a very small extent. Alloys with a high content of chromium and nickel (for example, 310, 310S, 314) have an almost stable austenitic structure and do not turn into ferrite or become magnetic when cold worked. Austenitic stainless steels are widely used in a variety of applications, including construction and automotive manufacturing, and as a structural material in the food and chemical industries.
P 0.002, Si 0.001, H 0.0003. Alloying with manganese (up to 2%) increases the mechanical properties of such iron and lowers the threshold of cold brittleness (Fig. 71-73).
Alloying with manganese and zinc leads to an increase in core. corrosion resistance of alloys. The mechanical properties of magnesium and its alloys are improved by alloying with copper, tin, zirconium, silicon and cerium.
Good casting properties make it possible to produce parts with complex profiles. Homogenizing annealing at 1050° C. Alloying with manganese increases the strength and toughness of the metal. Parts of compressors for liquefying gases and shut-off valves operating at low temperatures. Up to 700° C, cast iron ChN11G7H2Sh - up to 750° C
grains (former austenite grains). Alloying with manganese, chromium, and nickel, as well as an increase in phosphorus content, contribute to the development of reversible O. ch. With. The addition of 0.3-0.5% Mo reduces reversible O. x. With. For these purposes, molybdenum is sometimes replaced with tungsten in an amount of 1-1.2%, although its effect is less effective. The development of reversible temper embrittlement is greatly influenced by steel smelting technology, therefore different metallurgical methods. melts have different sensitivity to temper brittleness. Despite the huge amount of research and a number of interesting hypotheses put forward, physical. nature O. x. With. is not yet completely clear. Specialist. Using etchants, it is possible to establish significant changes in etchability at grain boundaries on steel subject to temper brittleness. There is reason to believe that the thermal brittleness of steel, which occurs during prolonged operation at temperatures of 450-550° and O. x. s., of the same origin.
The Cu-Al-Mn system is more promising for the development of new alloys. This position is based on a number of positive properties of manganese as an alloying component. The introduction of manganese into aluminum bronzes increases their strength and improves technological properties. Alloying with manganese also helps to increase the resistance of alloys against cavitation destruction and the most complete deoxidation of copper in the process of smelting bronze. The chemical compositions and mechanical properties of bronzes of the Cu-Al-Mg system, most widely used in domestic and foreign industry, are given in Table. I. 35. It should be noted that foreign alloys of the Cu-Al-Mn system are practically no different in composition from domestic bronze Br. AMts9-2. Thus, in world industry, alloys that lie on the state diagram of the Cu-Al-Mn system in the region of increased aluminum content with a lower, limited manganese content have found use. In this regard, at present it is premature to consider that from the point of view of finding high-strength alloys, the Cu-Al-Mn system is completely exhausted for further research. Of particular interest is the study of the properties of alloys with a high content of manganese, which has a positive effect on the level of mechanical and technological properties of alloyed bronzes. Aluminum bronzes with a high manganese content can obviously find application as new casting and wrought alloys. At the same time, for the most methodologically correct research, a more specific understanding of the copper angle of the phase diagram of the Cu-Al-Mn system is necessary.
The limited use of manganese as an alloying element in powder metallurgy is associated with the great difficulty of reducing it from oxides. Sintering of manganese-containing powder compositions must be carried out at temperatures of 1200 ... 1300 °C. Joint alloying with manganese and chromium significantly changes the mechanical characteristics of the part. Steel containing 0.6% carbon, 2% manganese and
There is great potential for increasing the performance properties of manganese surfacing due to heat treatment. A significant effect of dispersion hardening was discovered - spheroidization of primary and precipitation of secondary carbides. Alloying boron surfacing alloys with manganese is effective. In this case, the amount of brittle cementite decreases due to the formation of Fe0.4Mn3,bC carbide against the background of Fe2B borides. However, to austenitize the alloy and increase its impact resistance, additional alloying with nickel is necessary. The increase in the amount of austenite when alloying with manganese is unequal in effect to that achieved by alloying with nickel.
Being weakly oxidized, coatings of this type allow the molten metal to be alloyed with elements with a high affinity for oxygen. Alloying with manganese and silicon, carried out during their transition from ferromanganese and ferrosilicon to the weld pool, gives the joint high strength. In addition, metal powders can be added to the coating for alloying. The presence in it of a large amount of calcium compounds that bind sulfur and phosphorus well, which are then released into slag, ensures high purity of the deposited metal with a low content of sulfur and phosphorus. At high temperatures, fluorspar decomposes to release atomic fluorine, which binds hydrogen into a stable, metal-insoluble HF molecule. As a result, the deposited metal contains a small amount of hydrogen (4... 10 cm3 per 100 g of metal). The use of active deoxidizers (titanium, aluminum and silicon) in the coating ensures a low oxygen content in the weld metal (less than 0.05%). Therefore, the deposited metal is little prone to aging, is resistant to the formation of crystallization cracks and is ductile at low temperatures.
We recommend that you read
: Linear summation Linear viscoelastic Linear increase Linearity of equations Linearization of equations Linearized equation Sheet material Foundry heat-resistant Laboratory bench Literature dedicated Literature found Literary typeface Logarithmic dependence Logarithmic decrements Logarithmic normal