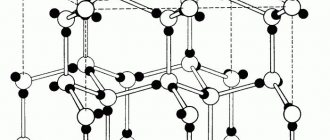
A striking example of allotropy is iron, which forms, depending on temperature, four main allotropic modifications, which are called: α-Fe, β-Fe, γ-Fe, δ-Fe.
Allotropic forms α-Fe - alpha iron, β-Fe - beta iron and δ-Fe - sigma iron have a crystal lattice in the shape of a centered cube (Fig. 1, a). The allotropic form of γ-Fe - gamma iron has a crystal lattice in the shape of a cube with centered faces (Fig. 1, b).
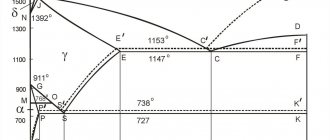
The transition of iron from one form to another - when cooled, it releases heat, and when heated, it absorbs heat. This is noted on the graphs of cooling or heating of iron. In Fig. Figure 2 shows a schematic graph of the cooling of pure iron.
Photo of iron crystal lattices
The crystal lattice is the spatial arrangement of atoms or ions in a crystal.
The points of the crystal lattice at which atoms or ions are located are called lattice nodes. Crystal lattices are divided into molecular, atomic, ionic and metallic.
On this curve, during transitions from one allotropic form to another, areas of constant temperatures are observed, namely: at t = 1535° - solidification of iron with the formation of δ-Fe;
photo of iron crystal lattices
at t = 1390°—transition δ-Fe - γ-Fe;
at t=898° - transition γ-Fe - β-Fe;
at t=775° - transition β-Fe - α-Fe.
When heating iron, the transformations occur in the reverse order, and the transition β -Fe - > γ-Fe occurs at t = 910°. In γ-Fe, the atoms are located more closely than in β-Fe, therefore the transition of γ-Fe to β-Fe is accompanied an increase in volume, and vice versa, the transition of β-Fe to γ-Fe is accompanied by a decrease in volume. The γ-Fe phosphorus is not magnetic.
The α-Fe form is magnetic. The β-Fe form is not magnetic, but has the same crystal lattice as the magnetic α-Fe form - therefore, metallographically, the β-Fe form is identified with the α-Fe form and I conditionally unite both forms under the same name α-Fe.
The main role in the technological processes of hot mechanical and thermal processing of iron-carbon alloys is played by: α-Fe and γ-Fe.
Rice. 2 Iron cooling curve
The property of γ-Fe to form solid solutions with carbon is important for heat treatment. The highest solubility of carbon in γ-Fe (up to 1.7%) is observed at t=1130°. With increasing and decreasing temperature from t = 1130°, the solubility of carbon in γ-Fe decreases.
The solid solution of carbon and other elements in γ-Fe is called austenite. α-Fe does not form stable solid solutions with carbon, like austenite. The solubility of carbon in α-Fe is negligible. Solid solutions of small amounts of C and other elements in α-Fe are called ferrite.
In addition to solid solutions of carbon in iron, iron-carbon alloys contain a chemical compound of iron and carbon - iron carbide Fe3C, which is called cementite. Cementite contains C - 6.67%. Austenite and ferrite are distinguished by ductility, ferrite, in addition, is soft. Cementite is extremely hard and brittle.
APPLICATION
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production. Iron is the main component of steels and cast irons - the most important structural materials. Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc. Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller. The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors. Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards. Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction. Iron is used as an anode in iron-nickel batteries and iron-air batteries. Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
Source
PROPERTIES
In its pure form under normal conditions it is a solid. It has a silver-gray color and a pronounced metallic luster. The mechanical properties of iron include its level of hardness on the Mohs scale. It is equal to four (average). Iron has good electrical and thermal conductivity. The last feature can be felt by touching an iron object in a cold room. Because this material conducts heat quickly, it removes most of it from your skin in a short period of time, which is why you feel cold. If you touch, for example, wood, you will notice that its thermal conductivity is much lower. The physical properties of iron include its melting and boiling points. The first is 1539 degrees Celsius, the second is 2860 degrees Celsius. We can conclude that the characteristic properties of iron are good ductility and fusibility. But that's not all. Also, the physical properties of iron include its ferromagnetism. What it is? Iron, whose magnetic properties we can observe in practical examples every day, is the only metal that has such a unique distinctive feature. This is explained by the fact that this material is capable of magnetization under the influence of a magnetic field. And after the end of the action of the latter, the iron, the magnetic properties of which have just been formed, remains a magnet for a long time. This phenomenon can be explained by the fact that in the structure of this metal there are many free electrons that are able to move.
STRUCTURE
Several polymorphic modifications have been established for iron, of which the high-temperature modification - γ-Fe (above 906°) forms a lattice of a face-centered cube of the Cu type (a0 = 3.63), and the low-temperature modification - the α-Fe lattice of a centered cube of the α-Fe type (a0 = 2.86).
Depending on the heating temperature, iron can be found in three modifications, characterized by different crystal lattice structures:
In the temperature range from the lowest to 910°C - a-ferrite (alpha ferrite), which has a crystal lattice structure in the form of a centered cube;
In the temperature range from 910 to 1390°C - austenite, the crystal lattice of which has the structure of a face-centered cube;
In the temperature range from 1390 to 1535 ° C (melting point) - d-ferrite (delta ferrite). The crystal lattice of d-ferrite is the same as that of a-ferrite. The only difference between them is the different (larger for d-ferrite) distances between the atoms.
When liquid iron is cooled, primary crystals (crystallization centers) appear simultaneously at many points in the cooled volume. With subsequent cooling, new crystalline cells are built around each center until the entire supply of liquid metal is exhausted.
The result is a granular structure of the metal. Each grain has a crystal lattice with a certain direction of its axes.
With subsequent cooling of solid iron, during the transition of d-ferrite to austenite and austenite to a-ferrite, new crystallization centers may appear with a corresponding change in grain size.
ORIGIN
Origin telluric (terrestrial) iron is rarely found in basalt lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both locations, pyrrhotite (Fe1-xS) and cohenite (Fe3C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO)n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises during reduction reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons. Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.
Additional information:
| 513 | Lattice parameters | 2.866 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 460 K |
| 516 | Name of space symmetry group | Im_ 3m |
| 517 | Symmetry space group number | 229 |
| 521 | Crystal grid #2 | γ-iron (austenite) |
| 522 | Lattice structure | Cubic face centered |
| 523 | Lattice parameters | 3.656 Å |
| 524 | c/a ratio | |
| 525 | Debye temperature | |
| 526 | Name of space symmetry group | Fm_3m |
| 527 | Symmetry space group number | 225 |
| 531 | Crystal grid #3 | δ-iron |
| 532 | Lattice structure | Cubic body-centered |
| 533 | Lattice parameters | 2.93 Å |
| 534 | c/a ratio | |
| 535 | Debye temperature | |
| 536 | Name of space symmetry group | Im_ 3m |
| 537 | Symmetry space group number | 229 |
| 900 | additional information | |
| 901 | CAS number | 7439-89-6 |
Iron
Pure iron (99.97%), purified by electrolysis
Iron
- a malleable silver-white metal with high chemical reactivity: iron quickly corrodes at high temperatures or high humidity in the air. Iron burns in pure oxygen, and in a finely dispersed state it spontaneously ignites in air. Denoted by the symbol Fe (Latin Ferrum). One of the most common metals in the earth's crust (second place after aluminum).
General information:
| 100 | General information | |
| 101 | Name | Iron |
| 102 | Former name | |
| 103 | Latin name | Ferrum |
| 104 | English name | Iron |
| 105 | Symbol | Fe |
| 106 | Atomic number (number in table) | 26 |
| 107 | Type | Metal |
| 108 | Group | Amphoteric, transitional, ferrous metal |
| 109 | Open | Known since ancient times |
| 110 | Opening year | before 5000 BC |
| 111 | Appearance, etc. | Malleable, ductile metal of silver-white color with a grayish tint |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | 5 allotropic modifications of iron: |
— α-iron (ferrite) with a cubic body-centered crystal lattice and ferromagnetic properties,
— β-iron with a cubic body-centered crystal lattice, differing from α-iron in the parameters of the crystal lattice and paramagnetic properties. β-iron serves to designate α-iron above the Curie point (Curie point of iron 769 °C),
— γ-iron (austenite) with a cubic face-centered crystal lattice,
— δ-iron with a cubic body-centered crystal lattice,
— ε-iron with a hexagonal close-packed crystal lattice
Crystal lattice of iron at room temperature
Rice. 3. Iron crystal lattice
The basic building blocks of solids such as salt and ice are molecules. Each molecule consists of two or more atoms, for example, sodium + chlorine (NaCl), as in table salt, and hydrogen + oxygen, as in ice (H2O). In metals, however, these building blocks are individual metal atoms: iron (Fe) atoms in an iron rod or copper (Cu) atoms in copper wire. Each grain in Figure 1 is what is called a crystal. In a crystal, which consists of atoms, all the atoms are uniformly arranged in layers. As shown in Figure 2, if you draw lines that connect the centers of the atoms, three-dimensional rows of small cubes will fill the entire space occupied by an individual grain. This three-dimensional structure is called a crystal lattice of atoms.
In iron at room temperature, these cubes have atoms at each of the eight corners and one atom right in the center of the cube. This crystal lattice is called body-centered, and the geometric arrangement of atoms is called a body-centered lattice. Iron with a body-centered crystal lattice is called ferrite
. Another name for ferrite is alpha iron or α-iron.
Powder metallurgy
Technology for obtaining metal powders and manufacturing products from them, as well as from compositions of metals with non-metals. In conventional metallurgy, metal products are produced by processing metals using methods such as casting, forging, stamping and extrusion. In powder metallurgy, products are produced from powders with particle sizes from 0.1 microns to 0.5 mm by cold pressing molding and subsequent high-temperature treatment (sintering). Powder metallurgy is economical in terms of materials and, like traditional metalworking methods, produces parts with the desired mechanical, electrical and magnetic properties. Powder metallurgy products are used in a variety of industries, including aerospace, electronics and transportation.
TECHNOLOGY.
The technological process of manufacturing products from metal powders consists of the following operations: preparing the mixture for molding, molding blanks or products and their sintering. The molding of blanks or products is carried out by cold pressing under high pressure (30–1000 MPa) in metal molds. Sintering of products from homogeneous metal powders is carried out at a temperature of 70–90% of the melting temperature of the metal. In mixtures, maximum cohesion is achieved near the melting point of the main component, and in cemented carbides - near the melting temperature of the binder. With increasing temperature and increasing sintering duration, shrinkage and density increase and contacts between grains improve. To avoid oxidation, sintering is carried out in a reducing atmosphere (hydrogen, carbon monoxide), in an atmosphere of neutral gases (nitrogen, argon) or in a vacuum.
APPLICATION.
The range of products manufactured using powder metallurgy methods is very wide and is constantly expanding. These include gears, levers, cams and pistons for the automotive, mechanical engineering, energy, communications, construction, mining and aerospace industries. Coins (for example, the Canadian nickel) are made from a strip obtained by cold rolling of nickel powder. Aluminum powder serves as a component of cellular concrete, paints and pigments.
Date added: 2018-08-06; ; We will help you write your work!
Source