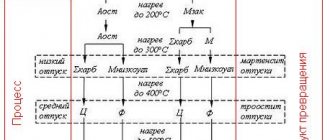
The role of austenite grain boundaries
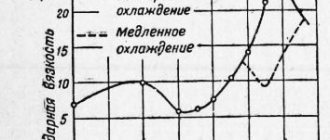
When austenite transforms into any other phase upon cooling, this new phase always first forms or nucleates at the old austenite grain boundaries. For example, if U8 pearlitic steel is first heated to full austenite at a temperature of 850 °C and then cooled in air to 650 °C, then small pearlite grains will form at the old austenite grain boundaries immediately after the temperature drops below 727 °C. When the temperature reaches 650 °C, these grains will begin to grow, but some austenite will still remain between them. If the sample continues to be kept at 650 °C, then the pearlite grains will grow until all the austenite is gone.
Influence of the degree of supercooling of austenite
Let us also consider an alternative heat treatment, when the sample is cooled from a temperature of 850 °C in a hot liquid at a temperature of 650 °C. This treatment ensures that the sample is cooled to a temperature of 650 °C much faster than when cooled in air. The sequence of austenite decomposition will be the same - small pearlite grains nucleate at the old boundaries of austenite grains and grow into the remaining austenite until it is all gone.
What changes compared to air cooling? Faster cooling will make three differences:
- pearlite grains will grow faster;
- the distance between the cementite plates in perlite will be much smaller;
- pearlite grains will be smaller, since more of them will nucleate at the boundaries of austenite grains.
General information about martensite
The structure based on a supersaturated solid solution of carbon in iron is called martensite. It is obtained by supercooling the austenite phase. In other words, martensite is the result of hardening steels with a carbon content above 0.3%. Martensite crystals have a tetragonal structure, where iron atoms occupy space at lattice sites.
In appearance, martensite consists of multiple dark iron needles on a light background. The angle of inclination of these needles is on average 60 degrees relative to each other. It is impossible to detect traces of carbon on the surface of martensite, since it is completely in a dissolved state.
Martensite is distinguished by its strength compared to other phases. Mechanical properties up to a certain point are directly dependent on the amount of carbon in the steel. But it is worth noting that after passing a certain point, the strength drops and fragility begins to increase.
According to research conducted in the 30s of the last century by Soviet scientists, the reasons for the high mechanical characteristics of martensite lie in the following:
- The structure of martensite is blocky in nature, despite the fact that the blocks themselves are quite small in size.
- Resistance to static distortion, which means the stability of the position of atoms when they are displaced from the ideal arrangement of atoms in the crystal lattice.
- When exposed to mechanical loads, and as a result of plastic deformation, tiny solid particles are released, blocking the sliding of the layers relative to each other and increasing the hardness of the alloy.
The hardness of martensite has a volatile character and depends on the heating, cooling and holding time of the steel. On average, its value ranges from 35 to 70 units on the Rockwell scale. Martensite also has a large specific volume. Its value is higher compared to other phase structures such as austenite, pearlite, etc.
As a consequence of all of the above, the formation of martensite is accompanied by significant changes in steel volume. This, in turn, leads to an undesirable increase in internal stress in the structure, which can cause cracks in the future.
Pearlite growth and martensite growth
Now suppose that U8 steel is cooled in a tank of water at an even lower temperature, for example, at room temperature. At such a low cooling temperature, one can expect the formation of martensite and significant strengthening of the steel. What are the similarities and differences between the formation of martensite at room temperature and pearlite at 650 °C? Like pearlite, martensite will begin to form along the boundaries of austenite grains, but unlike pearlite, martensite grows into austenite at an enormous rate. Pearlite grows into austenite at a rate of approximately 50 µm/s at 650 °C and even more slowly at higher temperatures. Martensite, on the other hand, grows into austenite at almost the speed of sound of 4510 m/s (in steel, not in air) at any temperature at which it is formed. In addition, in contrast to pearlite, which completely replaces austenite simply by holding the sample for a sufficiently long time at a low temperature.
Application of martensitic steel grades
Alloying elements are added to martensitic steels to obtain the desired properties of the alloys: strength, wear resistance, cold-heat resistance, corrosion resistance. One grade of alloy steel can contain up to 7 alloying elements. Steels are alloyed with nickel, chromium, nitrogen, tungsten, beryllium, vanadium, silicon, molybdenum, copper, and boron.
Usually, the designation of steel encodes alloying additives and their quantity (38ХН3МФА), some experimental ones are encrypted with the letter E. In this case, the letter does not reflect the composition of the steel - EI, EP3. Sometimes steels intended for the manufacture of aircraft and automobile exhaust valves are abbreviated as silchrome.
Alloyed martensitic steels are able to withstand aggressive environments: acids, alkalis, salts, aggressive gases. According to their application, martensitic steels are classified as corrosion-resistant, heat-resistant, heat-resistant and special-purpose steels.
Corrosion-resistant steel grades (15Х28, 20Х13, 12Х18Н9) are used in pilot production and in the chemical industry.
Heat-resistant steel grades (KhN60Yu, 12Kh25N16G7AR, (15Kh6SYu)) are used for the manufacture of parts that operate under moderate loads at temperatures up to 1000 degrees.
Products made from heat-resistant steel grades (15Х6СУ, 08Х13, 14Х17Н2) can operate under load for a very long and long period at high temperatures.
Special steels include steels from which armored sandwiches are rolled. A special place is occupied by Hadfield steel (1.1% carbon, 13% magnesium). When working under high pressure conditions, spontaneous plastic deformation occurs and, accordingly, the degree of its strength increases. The unique mechanical properties have not yet been fully studied.
Magnetic properties of martensitic steel
The martensitic structure of the steel lattice has pronounced magnetic properties. Martensite is a ferromagnet in its pure form. However, maintaining an ideal chemical composition is difficult. Carbon martensitic steels alloyed with molybdenum, cobalt and chromium (EX9K15M2), cobalt and chromium (EX5K6), chromium (EX3) can be classified as hard magnetic materials.
Alloying with cobalt is most effective from the point of view of magnetism - cobalt atoms have a magnetic moment, thus, the residual induction of martensite increases. Low price and ease of mechanical and heat treatment makes it possible to use martensitic steels in magnetic systems as switches for changing direction when supplying control signals.
Weldability of martensitic steels
Welding technologies for martensitic alloys are complicated by the increased fragility of the metal after hardening. These types of steel are cooked after preheating from approximately 200 to 450 degrees, the ambient temperature should not be negative. Typically, parts made of martensitic steel are welded using manual arc welding methods using electrodes coated with special compounds. Sometimes other types of welding are used: argon arc, electroslag, submerged arc.
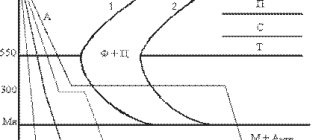
Martensitic transformation temperature range
Martensite will not replace all austenite until the quenching temperature becomes below the temperature, which is called the temperature of the end of the martensitic transformation Mk.
Moreover, martensite will not begin to form at all until the quenching temperature drops below the temperature at which the martensitic transformation of Mn begins.
If the quenching temperature is between Mn and Mk, then only part of the austenite will turn into martensite, and the remaining austenite - retained austenite - will continue to be in the steel.
The figure below is a graph that illustrates the dependence of the amount of martensite formed on the quenching temperature.
Figure - Dependence of the amount of martensite on the quenching temperature
The M50 symbol indicates the temperature at which 50% martensite is formed. If steel is cooled to a temperature of M50, then 50% of the austenite in it will turn into martensite. Moreover, this will happen within milliseconds after reaching the temperature M50. However, the remaining 50% of austenite that surrounds the martensite will remain as retained austenite until the temperature changes.
Martensite – metastable phase
At high cooling rates, a new phase appears - martensite, which is not predicted by the phase diagram. Such phases are called metastable, that is, unstable. If martensite is heated, as in the tempering process, it passes to more stable phases. These stable phases should already be on the phase diagram - heating martensitic structures to a temperature below A1 transforms both martensite and retained austenite into a mixture of ferrite and cementite.
The temperature at which martensitic transformation begins in steels depends very much on the carbon content of austenite.
Martensitic transformations in polymorphic crystals
Similar martensitic transformations, when atoms do not change places, but only shift relative to each other at distances smaller than interatomic ones (shortening interatomic bonds and changing the angles between them), are observed not only in iron alloys, but also in other polymorphic crystals.
Such transformations, also called metamorphoses, take place in steels, pure metals: iron, cobalt, titanium, lithium, at least 35 metals, in solid solutions based on them, in semiconductors and polymers, in intermetallic compounds.
In contrast to normal equilibrium polymorphic transformations, martensitic transformations are diffuseless and metastable. These transformations are of a non-equilibrium nature. The physics of metals says: nonequilibrium states must be self-organized.
From the point of view of the second law of thermodynamics, martensitic transformations in substances occur with a decrease in entropy. This means that the crystal structures of such transformations are the result of self-organization, and their parameters approach supercritical ones.
The structure of the intermetallic nickel monoaluminide after martensitic transformation is capable of withstanding temperatures up to 1300 degrees under high loads, but due to increased fragility it is used only as a heat-resistant coating for gas turbine engines.
Some intermetallic compounds with martensitic structures containing platinum are used as catalysts in the production of nitrogen. In connection with the tightening of environmental standards for cars, developments are underway to burn combustion products using intermetallic compounds.
On crystals of some semiconductors (silicon, germanium) one can observe direct or reverse diffuseless phase transitions of states. Experiments on heat treatment of silicon wafers were implemented in production with a 20% economic effect.
By studying the process of reversibility of martensitic transformations on recrystallization of the TiNi alloy (intermetallic compound), a change in the size of the samples was discovered.
Memory effect
Further experiments with various materials showed that many polymorphic crystals can exhibit properties such as shape memory effect, superelasticity and superplasticity.
Deformation and its reduction or even complete restoration of the original shapes during the reverse occurrence of martensitic transformations is called the shape memory effect. And all phenomena associated with martensitic transformations in substances are united under one name “unusual physical and mechanical properties.”
The shape memory effect is already used today in hydraulic couplings in shipbuilding and aviation, in damping devices, in thermal relays, in medicine for the treatment of scoliosis, joining broken bones, in heart surgery, and in dentistry.
Martensitic transformation and carbon content
Figure 1 shows the temperatures of the beginning and end of the martensitic transformation Mn and Mk for ordinary carbon steels depending on the carbon content. The temperature Mk has a fairly significant spread. Steel is most often hardened in water at room temperature. This temperature is marked by the horizontal line Troom. This line allows you to estimate at what carbon content complete hardening is possible at room temperature or how much retained austenite will be in the steel after hardening. According to Figure 1, quenching at room temperature becomes incomplete even at a carbon content of 0.3-0.4%, since at such a carbon content the temperature T drops below room temperature.
Figure 1 – Dependence of the temperatures of the beginning Tn and the end Tk of martensitic transformation on the carbon content in austenite
Retained austenite and carbon content
The percentage of retained austenite in steel is determined by X-ray method. Figure 2 shows the results of such measurements of the volume fraction of retained austenite in hardened ordinary carbon steels depending on the carbon content in them. Just like the temperature Tk, there is a large scatter of data. For example, for steel with a carbon content of 1.4%, the percentage of retained austenite ranges from 28 to 45%.
Figure 2 – Volume fraction in percent of retained austenite depending on carbon content for ordinary carbon steels hardened to room temperature
The graph in Figure 2 allows us to draw the following conclusions: 1) Steels with full lath martensite (carbon content less than 0.6%) will not have a significant amount of retained austenite. 2) Steels with fully lamellar martensite (carbon content greater than 1%) will have a significant amount of retained austenite. The higher the carbon content, the greater the amount of retained austenite.
Hardened steel type U8 with a carbon content of 0.77% will have a mixed lath-plate martensite structure and contain 6-10% retained austenite. Generally speaking, it is very difficult to see retained austenite between martensitic plates in an optical microscope until its content becomes about 10%.
Structure and properties of martensite
The word “martensite” is not known to anyone except metallurgists, but the history of its appearance is quite fascinating and deserves at least a few lines.
One of the great periods of change in civilization began when iron replaced bronze. However, it was completely unknown what exactly gave the metal its valuable properties, and for centuries the methods for producing high-quality steel were kept in almost alchemical secrets. Obviously, iron was the main component, but many other minor additives were empirically discovered using exotic methods of cooling red-hot metal to room temperature.
Iron finally replaced bronze after about 1200 BC. It was then discovered that pure iron is not a very useful material: it is soft and rusts easily. True, in India, near Delhi, there is a so-called Kutub column, which in some unknown way was made of pure iron, and it really exhibits high corrosion resistance, but that’s a completely different story.
By dissolving carbon in iron, you can get hard, but also very brittle cast iron, so this method of hardening is not suitable. Therefore, the search for the best technology to increase hardness while maintaining satisfactory ductility continued.
The technique of hardening iron in cold water was first mentioned in Homer's Odyssey. To blind a cyclops named Polyphemus, who imprisoned Odysseus, a red-hot stake was used, which was immediately dipped into cold water after the execution of Polyphemus. In modern terms, the iron stake was hardened and then tempered. As a result of rapid cooling, the carbon atoms stretched along the axis, increasing not only the hardness, but also the resistance of the metal to cracks - a martensitic transformation occurred in the structure.
But not everything turned out to be so simple. As chemical analysis processes developed, it became clear that different steel alloys with very similar compositions had completely different mechanical properties. Chemistry was unable to solve this riddle; the answer came from a completely different field of science - optics.
In 1890, Adolf Martens, a German specialist in the field of metallography, while studying the microstructure of steel, discovered many different patterns invisible to the naked eye. He found that harder steels have banded regions consisting of differently oriented microcrystalline phases, while lower quality materials have a weak coherent structure. Such patterned ones were called martensitic in honor of their discoverer.
The realization that the microscopic formation of steel's structure could be as important as its composition was a watershed moment in metallurgy. Thus, a new scientific direction was born - metallography; hundreds of materials with martensitic morphology were discovered and studied.
Martensite morphology is a term used by metallurgists to describe the study of the shape, size, texture, and phase distribution of physical objects. Martensite can be observed in the microstructure in two very different forms - lath or plate - depending on the carbon content.
Lamellar (twinned) martensite
Lamellar martensitic structures have increased strength but tend to be more brittle. In micrographs obtained using electron microscopes, regions of such martensite, which have the shape of lenses, are clearly visible. For steels containing less than 0.60% carbon, long thin plates of twinned martensite are often grouped in stacks.
As the percentage of carbon increases, so-called high-carbon martensite twins begin to replace dislocations within the plates. This transformation is accompanied by volumetric expansion, creating residual stresses (in addition to strains) due to the intercalation of solute atoms. The level of internal stresses that contribute to increasing the hardness of steel should not exceed its strength limit, otherwise cracking of the steel product is likely.
Lath (dislocation) martensite
Lath martensite is associated with high toughness and ductility, but at the same time with low strength. In low-carbon steels, lath martensites contain high-density dislocations as a substructure.
Many lath martensites consist of a twinned substructure rather than high-density dislocations. In addition, nano-sized precipitates are found at the twin interfaces, adhering to the ferrite matrix. This phenomenon is typical not only for steels, but also for titanium alloys, as well as other metals that have a body-centered crystal lattice.
Martensitic transformation in steels
To form martensite, the metal must be heated to the austenite state and quenched from the austenite phase to avoid the formation of pearlite. The quenching rate must be high enough to avoid bending the time-temperature-transformation curve - the so-called critical cooling rate. The formation of martensite involves the structural rearrangement (by shear displacement) of atoms from face-centered cubic austenite to a body-centered tetragonal martensite structure. This change is accompanied by a large increase in volume and leads to a state with a high level of internal stress. Therefore, martensite has a higher hardness than austenite of the same chemical composition.
Martensitic transformation, although not instantaneous, occurs much faster than diffusion-controlled processes. Among these processes is the formation of ferrite and pearlite. Their chemical composition differs significantly from the austenite from which they originate. Thus, the martensite structure is a metastable, deformation-induced state in which steel is found. The resulting hardness is determined primarily by the percentage of carbon content.
The intensity of martensitic transformation also depends on the carbon content in the steel. An increase in this indicator in austenite reduces the martensitic transformation temperature, which leads to difficulties in converting all austenite to martensite. The temperature range of martensitic transformation is especially important during welding, since it determines the state of residual stresses in the workpieces being welded. These temperatures for each specific brand can be calculated, and for the most commonly used brands they are given in reference books.