Martensite and martensitic transformation in steels
Martensite is a supersaturated solid solution of carbon in α-iron (α-Fe). Read what austenite, cementite, ferrite and pearlite are here. When eutectoid steel (0.8% carbon) is heated above point A1, the original pearlite structure will transform into austenite. In this case, all the carbon present in the steel will dissolve in austenite, i.e. 0.8%. Rapid cooling at a supercritical rate (see figure below), for example in water (600 °C/sec), prevents the diffusion of carbon from austenite, but the fcc crystal lattice of austenite will rearrange into the tetragonal lattice of martensite. This process is called martensitic transformation. It is characterized by the shear nature of the restructuring of the crystal lattice at a cooling rate at which diffusion processes become impossible. The product of martensitic transformation is martensite with a distorted tetragonal lattice. The degree of tetragonality depends on the carbon content in the steel: the more it is, the greater the degree of tetragonality. Martensite is a hard and brittle structure of steel. Found in the form of plates, under a microscope they look like needles.
The hardening temperature for most steels is determined by the position of the critical points A1 and A3. In practice, the hardening temperature of steels is determined using steel graders. How to choose the hardening temperature of steel, taking into account points Ac1 and Ac3, read the link.
Steel hardening
Hardening is a heat treatment operation of metal. It consists of heating the metal to a critical temperature at which the crystal lattice of the material changes , or to a temperature at which the phase dissolves in the matrix, which exists at a low temperature.
It is important to understand:
- After reaching a critical temperature, the metal undergoes rapid cooling.
- After hardening, the steel acquires the structure of martensite (named after Adolph Martens) and therefore becomes hard.
- Hardening increases the strength of steel. The metal becomes even harder and more wear-resistant.
- A distinction should be made between conventional quenching of the material and quenching to obtain excess vacancies.
Hardening modes differ in the speed of the process and heating temperature. There are also differences in the duration of exposure at a given temperature and cooling rate.
Microstructure of steel after hardening
Most steels after hardening are characterized by the structure of martensite and retained austenite, the amount of the latter depending on the carbon content and the qualitative and quantitative content of alloying elements. For structural steels of medium alloying, the amount of retained austenite can be in the range of 3-5%. In tool steels this amount can reach 20-30%.
In general, the structure of steel after hardening is determined by the final requirements for the mechanical properties of the product. Along with martensite, after quenching, ferrite or cementite may be present in the structure (in case of incomplete quenching). When steel is isothermally hardened, its structure may consist of bainite. The structure, final properties and hardening methods of steel are discussed below.
Partial hardening of steel
Partial quenching is called quenching, in which the cooling rate is not sufficient for the formation of martensite and it turns out to be below critical. This cooling rate is indicated by the blue line in the figure. During partial hardening, the “nose” of the C-curve steel seems to be touched. In this case, in the structure of the steel, along with martensite, troostite will be present in the form of black island inclusions.
The microstructure of partially hardened steel looks something like this:
Partial hardening is a defect that is eliminated by complete recrystallization of the steel, for example, during normalization or during reheating for hardening.
Steel 55 structural carbon quality
Substitutes
- Steel 50,
- steel 60,
- steel 50G
Foreign analogues
Decoding
The number 55 means that the average carbon content in steel is 0.55%.
Characteristics and purpose
Steel grade 55 belongs to unalloyed special structural high-quality carbon steels, has high strength and high elastic properties, is used in the manufacture of parts after normalization with tempering and hardening with tempering that work for friction, for example:
- Gears,
- rolling rolls,
- stocks,
- heavily loaded shafts,
- axles,
- bandages,
- lightly loaded springs and leaf springs,
- plowshares,
- track link fingers
- gearbox clutches,
- injector housings
Chemical composition, % (GOST 1050-88)
| C | Si | Mn | Cr | S | R | Cu | Ni | As |
| no more | ||||||||
| 0,52-0,60 | 0,17-0,37 | 0,50-0,80 | 0,25 | 0,040 | 0,035 | 0,25 | 0,25 | 0,08 |
Chemical composition, % (GOST 1050-2013)
| steel grade | Mass fraction of elements, % | |||||||
| C | Si | Mn | P | S | Cr | Ni | Cu | |
| no more | ||||||||
| 55 | 0,52-0,60 | 0,17-0,37 | 0,50-0,80 | 0,030 | 0,035 | 0,25 | 0,30 | 0,30 |
Hardness HB (Brinnell) (GOST 1050-2013)
| steel grade | Hardness HB, no more, for metal products | |||
| hot rolled and forged | calibrated and with special surface finishing | |||
| without heat treatment | after annealing or high tempering | hard-worked | after annealing or high tempering | |
| 55 | 255 | 217 | 269 | 229 |
Use of steel 55 (GOST 1050) for oxygen fittings (according to GOST 12.2.052)
| Oxygen pressure, MPa (kgf/cm2), no more | In instrumentation shutdown fittings (DN ≤ 6) | ||||||||
| in shut-off valves | in control valves | ||||||||
| when managing | |||||||||
| local | remote | local | remote | ||||||
| frame | shutter parts | frame | shutter parts | frame | shutter parts | frame | shutter parts | frame | valve parts, spindle with locking cone ≥60° |
| 1,6 (16) | 0,6 (6) | 1,6 (16) | |||||||
NOTE. Fittings made of carbon steels and cast irons coated with organosilicate materials are equivalent to fittings made of stainless steels.
Heat treatment
Small parts made of grade 55 steel (up to 10-12 mm in diameter) are hardened in oil at a temperature of 820-860 ° C, larger parts in water at a temperature of 800-820 ° C, tempering is carried out at different temperatures depending on the required mechanical properties.
Springs of winches, swivels, core bits, valve springs after hardening are tempered at a temperature of 300-380 °C.
Temperature of critical points, °C
| Ac1 | Ac3 | Ar3 | Ar1 | Mn |
| 725 | 755 | 750 | 690 | 320 |
Mechanical properties of metal products (GOST 1050-2013)
| Mechanical properties, no less | |||
| Yield strength σ0.2, N/mm2 | Tensile strength σв, N/mm2 | Relative elongation δ5, % | Relative narrowing ψ, % |
| 380 | 650 | 13 | 35 |
Mechanical properties of metal products made from steel 35 depending on size (GOST 105-2013)
| Mechanical properties of metal products size | |||
| Yield strength σ0.2, MPa not less | Tensile strength σв, MPa | Relative elongation δ5, % | Impact work KU, J |
| no less | |||
| up to 16 mm incl. | |||
| 550 | 800-950 | 12 | + |
| St. 16 to 40 mm incl. | |||
| 490 | 750-900 | 14 | + |
| St. 40 to 100 mm incl. | |||
| 420 | 700-850 | 15 | + |
NOTE.
- The “+” sign means that the tests are carried out to collect statistical data, and the test results are recorded in the quality document.
- The mechanical properties of metal products made from grade 30 steel apply to metal products up to 63 mm in size inclusive.
- The values of mechanical properties are given for metal products with a round cross-section. For rectangular sections, the ranges of equivalent diameters are in accordance with Appendix B (GOST 1050-2013).
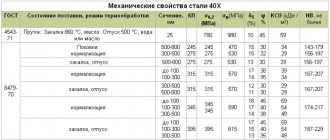
Mechanical properties of forgings (GOST 8479-70)
| Heat treatment | Section, mm | KP | σ0.2, MPa | σв, MPa | δ5, % | ψ, % | KCU, J/cm2 | Hardness HB, no more |
| no less | ||||||||
| Normalization | 100-300 | 315 | 315 | 570 | 14 | 35 | 34 | 167-207 |
| Hardening + tempering | Up to 100 | 490 | 490 | 655 | 16 | 45 | 59 | 212-248 |
Mechanical properties depending on the section
| Section, mm | σ0.2, MPa | σв, MPa | δ5, % | ψ, % | KCU, J/cm2 | Hardness HB |
| no less | ||||||
| Quenching at 840 °C in water; tempering at 400 °C, cool. on air | ||||||
| 20 | 870-990 | 1060-1210 | 7-8 | 43-52 | 54-67 | 341 |
| 40 | 640-740 | 900-1000 | 10-11 | 43-47 | 39-53 | 290 |
| 60 | 590-650 | 820-930 | 10-12 | 42-46 | 32-49 | 266 |
| Quenching at 840°C in water; tempering at 500°C, cool. on air | ||||||
| 20 | 710-800 | 900-1000 | 10-12 | 53-59 | 59-98 | 285 |
| 40 | 550-610 | 820-900 | 12-14 | 45-52 | 49-69 | 264 |
| 60 | 510-570 | 750-850 | 13-14 | 43-52 | 39-59 | 239 |
| Normalization at 830-860°C, cool. on air; holiday at 650-800°C, cool. with oven | ||||||
| Up to 100 | 325 | 650 | 12 | 35 | 29 | — |
| 101-300 | 315 | 630 | 11 | 28 | 25 | 170-229 |
| 301-500 | 305 | 610 | 10 | 25 | 25 | — |
| Normalization at 880-860 °C, cool. on air; holiday at 550-600 °C, cool. in air or with oven | ||||||
| Up to 1200 | Not defined | 215-265 | ||||
Mechanical properties depending on tempering temperature
| ttp, °С | σ0.2, MPa | σв, MPa | δ5, % | ψ, % | KCU, J/cm2 | Hardness HRCе |
| 200 | 1620-2210 | 1720-2280 | 2-3 | 7-12 | 5-10 | 64 |
| 300 | 1350-1790 | 1500-1940 | 3-4 | 20-25 | 5-10 | 56 |
| 400 | 1100-1380 | 1260-1590 | 4-5 | 31-36 | 19-49 | 47 |
| 500 | 820-1000 | 970-1120 | 7 | 41-45 | 39-78 | 40 |
| 600 | 600-700 | 800-890 | 10-11 | 51-54 | 69-108 | 30 |
NOTE. Hardening at 840 °C in oil.
Impact strength KCU
| Delivery status | KSU, J/cm2 at temperature, °C | ||
| +20 | -20 | -50 | |
| Hot rolled | 26 | 18 | 13 |
Endurance limit
| Heat treatment | σ-1, MPa | τ-1, MPa | n |
| Quenching at 790 °C in water; tempering at 650 °C, cool. on air | 377 | 219 | 106 |
Technological properties
Forging temperature, °C: beginning 1240, end 800. Sections up to 400 mm are cooled in air.
Weldability - not applicable to welded structures. CTS followed by heat treatment.
Cutting ability - Kv tv.alloy. = 1 and Kv b.st. = 0.65 in a normalized state at HB 212-225.
Flock sensitivity is insensitive.
Tendency to temper brittleness - not prone.
Hardenability
The hardenability band of steel 55 after quenching from 850 °C is shown in Fig. 5. 70 — Distance from the cooled end, mm Fig. 5. Steel hardenability band 55
Critical diameter d
| Amount of martensite, % | d, mm. after hardening | |
| in water | In oil | |
| 50 | 16-28 | 9-16 |
Type of delivery
- Long products, including shaped steel: GOST 1050-88, GOST 2590-88, GOST 2591-88, GOST 2879-88, GOST 8509-93, GOST 8510-86, GOST 8239-89, GOST 8240-89.
- Calibrated rod GOST 7417-75, GOST 8559-75, GOST 8560-78.
- Polished rod and silver GOST 14955-77.
- Thick sheet GOST 1577-93, GOST 19903-74.
- Tape GOST 2284-79. Strip GOST 103-76, GOST 1577-93, GOST 82-70.
- Forgings and forged blanks GOST 1133-71, GOST 8479-70.
- Rolls OST 24.013.21-85, GOST 5399-69, OST 24.013.04-83.
Density ρп kg/cm3 at test temperature, °С
| Steel | 20°C |
| 55 | 7280 |
Linear expansion coefficient α*106, K-1
| steel grade | α*106, K-1 at test temperature, °C | |||||||||
| 20-100 | 20-200 | 20-300 | 20-400 | 20-500 | 20-600 | 20-700 | 20-800 | 20-900 | 20-1000 | |
| 55 | 11,0 | 11,9 | 12,7 | 13,4 | 14,0 | 14,5 | 14,8 | 12,5 | 13,5 | 14,4 |
Thermal conductivity coefficient λ W/(m*K)
| Steel grade | λ W/(m*K), at test temperature, °C | |||||||||
| 20 | 100 | 200 | 300 | 400 | 500 | |||||
| 55 | — | 68 | 55 | — | 36 | 32 | ||||
Young's modulus (normal elasticity) E, GPa
| Steel grade | At test temperature, °C | |||||||||
| 20°C | ||||||||||
| 55 | 210 | |||||||||
Specific heat capacity c, J/(kg*K)
| steel grade | s, J/(kg*K), at test temperature, °C | |||||||||
| 20-100 | 20-200 | 20-300 | 20-400 | 20-500 | ||||||
| 55 | 479 | 487 | — | 525 | 571 | |||||
Find out more
Structural alloy steel 38ХС…
Steel 08Х15Н24В4ТР (EP164) heat-resistant austenitic…
Steel grade 03Х18Н11 chromium-nickel, austenitic…
Structural alloy steel 20ХНР...
Incomplete hardening of steels
Quenching at temperatures lying between A1 and A3 (incomplete quenching) retains in the structure of hypoeutectoid steels, along with martensite, part of the ferrite, which reduces the hardness in the quenched state and worsens the mechanical properties after tempering. This is understandable, since the hardness of ferrite is 80HRC, and the hardness of martensite depends on the carbon content and can be more than 60HRC. Therefore, these steels are usually heated to temperatures 30–50 °C above A3 (full hardening). In theory, incomplete hardening of steels is not permissible and is considered a defect. In practice, in some cases, incomplete quenching can be used to avoid quenching cracks. Very often this concerns hardening with high frequency currents. With such hardening, it is necessary to take into account its feasibility: type of production, annual program, type of product responsibility, economic justification. For hypereutectoid steels, quenching at temperatures above A1 but below Acm produces excess cementite in the structure, which increases the hardness and wear resistance of the steel. Heating above the temperature Acm leads to a decrease in hardness due to the dissolution of excess cementite and an increase in retained austenite. In this case, the austenite grain grows, which also negatively affects the mechanical characteristics of the steel.
Thus, the optimal quenching for hypoeutectoid steels is quenching from a temperature 30–50 °C above A3, and for hypereutectoid steels – at 30–50 °C above A1.
The cooling rate also affects the hardening result. The optimal cooling medium is one that quickly cools the part in the temperature range of minimum stability of supercooled austenite (in the range of the nose of the c-curve) and slowly in the temperature range of martensitic transformation.
Cooling stages during hardening
The most common quenching media are water of various temperatures, polymer solutions, alcohol solutions, oil, molten salts. When hardening in these environments, several cooling stages are distinguished:
— film cooling, when a “steam jacket” is formed on the surface of the steel;
- nucleate boiling, which occurs with the complete destruction of this steam jacket;
— convective heat transfer.
More details about the cooling stages during quenching can be found in the article “Characteristics of quenching oils”
In addition to liquid quenching media, cooling in a gas flow of different pressures is used. It can be nitrogen (N2), helium (He) and even air. Such quenching media are often used in vacuum heat treatment. Here it is necessary to take into account the fact of the possibility of obtaining a martensitic structure - the hardenability of steel in a certain environment, i.e. the chemical composition of the steel on which the position of the c-curve depends.
What is the hardening of metal?
The ancient blacksmiths knew that the effect of high temperature on metal can change its structure and properties and actively used this in practice. Subsequently, it was scientifically established that hardening of products made of steel, which involves heating and subsequent cooling of the metal, can significantly improve the mechanical characteristics of finished products, significantly increase their service life and even ultimately reduce their weight by increasing the strength of the part. What’s noteworthy is that hardening parts made from inexpensive steel makes it possible to give them the required characteristics and successfully use them instead of more expensive alloys.
The meaning of the process, which is called hardening of steel alloy products, is to heat the metal to a critical temperature and then cool it. The main goal pursued by this heat treatment technology is to increase the hardness and strength of the metal while simultaneously reducing its ductility.
There are various types of hardening and subsequent tempering, differing in modes of implementation, which determine the final result. Hardening modes include the heating temperature, the time and speed of its implementation, the time the part is kept in a state heated to a given temperature, and the speed at which cooling is carried out.
The most important parameter when hardening metals is the heating temperature, upon reaching which the atomic lattice is rearranged. Naturally, for different grades of steel, the critical temperature value is different, which depends, first of all, on the level of carbon content and various impurities in their composition.
After hardening, both the hardness and brittleness of the steel increases, and a layer of scale appears on its surface, which has lost a significant amount of carbon. The thickness of this layer must be taken into account when calculating the allowance for further processing of the part.
Iron-carbon phase diagram
When hardening products made of steel alloys, it is very important to ensure a given cooling rate of the part, otherwise the already rearranged atomic structure of the metal may go into an intermediate state. Meanwhile, too rapid cooling is also undesirable, as it can lead to the appearance of cracks on the part or to its deformation. In order to avoid the formation of such defects, the cooling rate after the temperature of the heated metal drops to 200 degrees Celsius is somewhat slowed down.
To heat parts made of carbon steel, chamber furnaces are used, which can heat up to 800 degrees Celsius. For hardening certain grades of steel, the critical temperature can be 1250–1300 degrees Celsius, so parts made from them are heated in a different type of furnace. The convenience of hardening steel of these grades lies in the fact that products made from them are not subject to cracking when cooled, which eliminates the need for preheating.
You should take a very responsible approach to hardening parts of complex configurations that have thin edges and sharp transitions. To prevent cracking and warping of such parts during the heating process, it should be carried out in two stages. At the first stage, such a part is preheated to 500 degrees Celsius and only then the temperature is brought to a critical value.
Heating of steel during hardening with high frequency currents
For high-quality hardening of steels, it is important to ensure not only the level of heating, but also its uniformity. If the part is massive or has a complex configuration, it is possible to ensure uniform heating only in several approaches. In such cases, heating is carried out with two delays, which are necessary so that the achieved temperature is evenly distributed throughout the entire volume of the part. The total heating time also increases if several parts are placed in the oven at the same time.
Factors influencing the position of c-curves:
- Carbon. Increasing the carbon content to 0.8% increases the stability of supercooled austenite, and accordingly the c-curve shifts to the right. When the carbon content increases above 0.8%, the c-curve shifts to the left;
— Alloying elements. All alloying elements increase the stability of austenite to varying degrees. This does not apply to cobalt; it reduces the stability of supercooled austenite;
— Grain size and homogeneity. The larger the grain and the more uniform its structure, the higher the stability of austenite;
— An increase in the degree of distortion of the crystal lattice reduces the stability of supercooled austenite.
Temperature affects the position of c-curves through all of the above factors.
Methods of hardening steels
In practice, various cooling methods are used depending on the size of the parts, their chemical composition and the required structure (diagram below).
Diagram: Cooling rates for different methods of hardening steels
Continuous hardening of steel
Continuous hardening (1) is a method of cooling parts in one environment. After heating, the part is placed in a quenching medium and left there until completely cooled. This technology is the most common and is widely used in mass production. Suitable for almost all types of structural steels.
Hardening in two environments
Quenching in two environments (speed 2 in the figure) is carried out in different quenching environments, with different temperatures. First, the part is cooled in the temperature range, for example, 890–400 °C, for example in water, and then transferred to another cooling medium - oil. In this case, the martensitic transformation will already occur in an oil environment, which will lead to a decrease in the leash and warping of steel. This hardening method is used for heat treatment of stamping tools. In practice, the opposite technological technique is often used - first the parts are cooled in oil and then in water. In this case, the martensitic transformation occurs in oil, and the parts are moved into water for faster cooling. This saves time on implementing the hardening technology.
Step hardening
During stepwise quenching (speed 3), the product is cooled in a quenching medium having a temperature higher than the martensitic transformation temperature. In this way, a certain isothermal holding is obtained before the transformation of austenite into martensite begins. This ensures uniform temperature distribution over the entire cross-section of the part. This is followed by final cooling, during which the martensitic transformation occurs. This method produces hardening with minimal internal stress. Isothermal holding can be done just below the temperature Mn, after the start of the martensitic transformation (speed 6). This method is more difficult from a technological point of view.
Isothermal hardening of steels
Isothermal hardening (speed 4) is done to obtain the bainitic structure of the steel. This structure is characterized by an excellent combination of strength and plastic properties. During isothermal hardening, parts are cooled in a bath of molten salts, which have a temperature 50–150 °C above the martensite point Mn, maintained at this temperature until the end of the transformation of austenite into bainite, and then cooled in air.
When hardening onto bainite, it is possible to obtain two different structures: upper and lower bainite. Upper bainite has a feathery structure. It is formed in the range of 500-350°C and consists of lath-shaped ferrite particles <1 µm thick and 5-10 µm wide, as well as thin cementite particles. The structure of upper bainite is characterized by higher hardness and strength, but lower ductility. Lower bainite has a needle-like martensite-like structure and is formed in the range of 350-200 °C. Lower bainite consists of fine particles of ε-carbides located in ferrite platelets. The bainite transformation never goes to completion. The structure always contains martensite and retained austenite. More preferable, in terms of performance characteristics, is the lower bainite structure. Products with such a structure are used in car construction, where parts experience shock-tensile stresses. The bainite hardening technology requires special hardening equipment. Additional materials on this technology can be found in the article “Technology of hardening for bainite.”
Cold treatment (5) is used for steels in which the temperature of the end of the martensitic transformation Mk is below room temperature.
High-speed steels, cemented parts, measuring instruments, and other particularly precise products are subjected to cold treatment. You can read more about this non-standard method of heat treatment in the article “Cold processing of steel parts”
Steel after hardening: structure and properties
Steel in its usual form is a fairly soft and malleable metal. Some grades do not require special strength (these are the so-called ordinary quality steels, produced in accordance with the requirements of GOST 380): the indicators obtained after smelting are quite sufficient, for example, for sewer manholes or protective gratings. But there are categories of steels - structural and instrumental - for which the initial strength indicators are not enough. They must be subjected to heat treatment. Its main type is hardening.
Microstructure of steel 45 after annealing and hardening
Hardening: the essence of the operation
As is known, any steel is a solid solution of carbon in the main structure of α-iron. In this case, the grade determines the percentage of carbon content (for example, the grade “65 steel” means that it contains 0.65% C, U13 steel contains about 1.3% C, and so on). However, this element is quite chemically active, therefore, during the smelting process (at 1600...2000 °C) it is actively bound by iron, resulting in the formation of Fe3C cementite. Everything else is ferrite - a fairly soft structural component. The large amount of ferrite in low-carbon steels causes their increased ductility, even in a cold state. This does not apply to steels:
- alloyed (they are produced according to the requirements of GOST 4543);
- bearings according to GOST 801;
- spring-spring according to GOST 2052 and GOST 14959;
- all types of instrumental, both alloyed and unalloyed.
To understand the effectiveness of hardening, it is necessary to refer to the structure of the steel after smelting and subsequent hot rolling into the required profile - strip, rod or special profile (angle, channel, etc.).
Any steel has a crystalline structure, which is made up of an infinite number of crystals. If steel is poured and the melt is then cooled, these crystals turn into multifaceted formations called grains. Since active saturation with oxygen occurs, voids appear between adjacent crystals, which, during the cooling of the ingot, are gradually filled with sulfur, phosphorus and other low-melting non-metallic inclusions. This not only reduces ductility (phosphorus and sulfur are very fragile chemical elements), but also contributes to the appearance of very coarse accumulations of grains, which makes the metal uneven in its density. It is impossible to process such products - the ingot will begin to split. Therefore, immediately after smelting, rolling is performed, during which the original defects are healed and the structure becomes more homogeneous. Accordingly, the density increases and surface cracks disappear.
Temperature of the workpiece depending on the color when heated
Plastic deformation has a positive effect only on the macrostructure. Hardening is responsible for changing the microstructure - a set of technological methods of heat treatment, the essence of which is to increase the strength characteristics of steel. The point of hardening is to fix a number of high-temperature components of the microstructure (giving steel resistance) for normal operating conditions of products. Accordingly, steel, without changing its chemical composition, will sharply increase the level of some of its mechanical characteristics:
- limit of temporary resistance σв, MPa;
- yield strength σt, MPa;
- fatigue limit σi, MPa;
- hardness according to Brinell HB or Rockwell HRC.
At the same time, some indicators - in particular, impact strength, relative elongation - become lower after hardening. If this is critical from the point of view of the subsequent operational durability of the part (and in most cases this is the case), then it is correct to perform a number of additional operations after hardening: tempering, aging, etc.
Dependence of martensite hardness on carbon content
The hardness of steel after quenching depends on the hardness of martensite, which in turn depends on the carbon content. As the carbon content increases, the hardness after hardening of the steel also increases. The graphical dependence is shown in the figure.
Graph of martensite hardness versus carbon content
Solvent for alkyd enamel - https://www.dcpt.ru
Temperature selection for hardening
The decision at what temperature to harden the metal is determined by the chemical composition of the steel.
There are two types of hardening:
- full;
- incomplete.
Based on the diagram of critical points, one can see that hypoeutectoid steel during the process of complete hardening should be heated above the Ac3 point by 30–50 degrees C. As a result, the steel will have a homogeneous austenite structure. Subsequently, under the influence of the cooling process, it will turn into martensite.
Figure No. 1. Critical points .
Incomplete hardening is more often used for tool steel. The purpose of incomplete hardening is to reach a temperature at which the process of formation of excess phases takes place. Heating of steel occurs in the temperature range from Ac1 - Ac2 . At the same time, some amount of ferrite remaining after hardening of the steel will remain in the martensite structure.
To harden hypereutectoid steel, it is better to maintain a temperature 20–30 degrees higher than Ac1 - incomplete hardening. Because of this, cementite will be retained during heating and cooling, which increases the hardness of martensite. When hardening, hypereutectoid steel should not be heated above the specified temperature. This may affect hardness.