Steel hardening
Hardening is a heat treatment operation of metal. It consists of heating the metal to a critical temperature at which the crystal lattice of the material changes , or to a temperature at which the phase dissolves in the matrix, which exists at a low temperature.
It is important to understand:
- After reaching a critical temperature, the metal undergoes rapid cooling.
- After hardening, the steel acquires the structure of martensite (named after Adolph Martens) and therefore becomes hard.
- Hardening increases the strength of steel. The metal becomes even harder and more wear-resistant.
- A distinction should be made between conventional quenching of the material and quenching to obtain excess vacancies.
Hardening modes differ in the speed of the process and heating temperature. There are also differences in the duration of exposure at a given temperature and cooling rate.
Temperature selection for hardening
The decision at what temperature to harden the metal is determined by the chemical composition of the steel.
There are two types of hardening:
- full;
- incomplete.
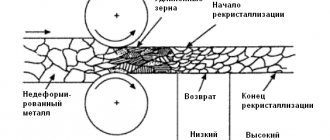
Based on the diagram of critical points, one can see that hypoeutectoid steel during the process of complete hardening should be heated above the Ac3 point by 30–50 degrees C. As a result, the steel will have a homogeneous austenite structure. Subsequently, under the influence of the cooling process, it will turn into martensite.
Figure No. 1. Critical points .
Incomplete hardening is more often used for tool steel. The purpose of incomplete hardening is to reach a temperature at which the process of formation of excess phases takes place. Heating of steel occurs in the temperature range from Ac1 - Ac2 . At the same time, some amount of ferrite remaining after hardening of the steel will remain in the martensite structure.
To harden hypereutectoid steel, it is better to maintain a temperature 20–30 degrees higher than Ac1 - incomplete hardening. Because of this, cementite will be retained during heating and cooling, which increases the hardness of martensite. When hardening, hypereutectoid steel should not be heated above the specified temperature. This may affect hardness.
Hardenability and hardenability. Hardening methods. Cooling media. Hardening defects.
Hardenability refers to the ability of steel to increase hardness
Calcination refers to the ability of steel to be hardened to a certain depth.
The depth of the hardened zone is a criterion for hardenability. Typically, parts have higher hardness on the surface and lower hardness in the core. This is explained by the thermal conductivity of steel. However, most parts must be baked through. To ensure through calcination and control on it, the term critical diameter was introduced. By which we mean the maximum cross-sectional dimension of a part that is calcined through. To do this, in order to harden the part through and through in this cooler, it is necessary that the critical diameter Dcr is greater than the cross-sectional diameter of the part.
Depending on the steel composition, shape and part, the hardening method is chosen. The main methods of hardening include: hardening in one cooler, intermittent hardening, isothermal hardening and various combinations of these methods.
Quenching in one cooler
- this is the most common method of hardening; it involves heating steel above the temperatures corresponding to the critical point Ac1 and Ac3, followed by holding and cooling at a rate above the critical one in one cooler (1). Water is used as a cooling medium for carbon and low-alloy steels, while oil is used for alloy steels. Some high-alloy steels are hardened in still air. The cross-sectional area of the part is also important; Thus, carbon and low-alloy steels with a cross-section of 5 mm are hardened in water. Parts with a variable cross-section or a cross-section of less than 5 mm can also be quenched in oil, since the cooling capacity of the oil is sufficient to anneal thin-section parts through and through. Disadvantage: the occurrence of large internal stresses and the formation of cracks.
Hardening in two environments
- this method consists in the fact that a part heated to the required temperature, maintained at this temperature, is transferred to a cooler that provides such a cooling rate that would prevent the decomposition of supercooled austenite in the temperature range of minimum stability of austenite, for example, into water, and then transferred to less an intensely cooling environment in which the hardening actually occurs (2). This method of hardening makes it possible to reduce the level of hardening stresses and prevent the occurrence of hardening defects such as warping. Disadvantage: requires highly qualified workers
Jet hardening
- this method is used when there is no need to harden the part to the same hardness over the entire surface. For such types of parts as a chisel, it is important to obtain a high hardness of the cutting edge while maintaining a tough shank; in this case, the tool, heated to a given temperature, is cooled from the working surface with jets of water, thereby destroying the “steam jacket” and the working surface of the tool is intensively cooled.
Tempering by self-tempering
- this method practically has the same functional purpose as jet quenching, for example, a chisel is heated to a given temperature and only the working part is transferred into a cooling medium, then after removal from the quenching medium, it is held in free air, as a result of which the working part is released due to heating from the non-working, uncooled part. The tempering temperature of the cooled part is controlled visually by the colors of tarnish. Hardening with self-tempering can be carried out using another method; for this, the part (tool) is completely hardened, and then only the non-working part is heated and the working part is tempered due to thermal conductivity.
Step hardening
- this method is a variation of the hardening method in two environments (2). However, from the point of view of part processing, a part with a variable cross-section is more efficient. When cooling to a temperature slightly above the point at which the martensitic transformation begins, it is necessary to equalize the temperature across all sections; to do this, hold it in the first cooler until the temperature is equalized, and then transfer the part to a second cooled environment, in which quenching occurs.
Isothermal hardening
— in contrast to stepwise isothermal hardening, the part is placed in a cooling environment with a temperature slightly higher than the temperature at which the martensitic transformation begins and is kept in this environment until the transformation is completely completed (4). As a result of isothermal hardening, a structure is formed - bainite, which, compared to martensite, has a slightly lower hardness and increased viscosity.
Cold processing of steel.
In hardened steel, especially with a carbon content of more than 0.4-0.5%, there is always residual austenite, which reduces hardness and wear resistance. To reduce the amount of retained austenite in hardened steel, cold treatment is used, which consists of cooling the hardened steel to temperatures below 0. Lowering temperatures to -30 - -70 degrees. For most steels, it causes the transformation of retained austenite into martensite, which increases hardness. But because At the same time, the stresses increase, then the products are cooled slowly and tempering is performed immediately after cold treatment. Cold treatment is used for measuring instruments, springs and parts made of case-hardened high-alloy steels, which retain a lot of austenite after hardening.
Hardenability refers to the ability of steel to increase hardness
Calcination refers to the ability of steel to be hardened to a certain depth.
The depth of the hardened zone is a criterion for hardenability. Typically, parts have higher hardness on the surface and lower hardness in the core. This is explained by the thermal conductivity of steel. However, most parts must be baked through. To ensure through calcination and control on it, the term critical diameter was introduced. By which we mean the maximum cross-sectional dimension of a part that is calcined through. To do this, in order to harden the part through and through in this cooler, it is necessary that the critical diameter Dcr is greater than the cross-sectional diameter of the part.
Depending on the steel composition, shape and part, the hardening method is chosen. The main methods of hardening include: hardening in one cooler, intermittent hardening, isothermal hardening and various combinations of these methods.
Quenching in one cooler
- this is the most common method of hardening; it involves heating steel above the temperatures corresponding to the critical point Ac1 and Ac3, followed by holding and cooling at a rate above the critical one in one cooler (1). Water is used as a cooling medium for carbon and low-alloy steels, while oil is used for alloy steels. Some high-alloy steels are hardened in still air. The cross-sectional area of the part is also important; Thus, carbon and low-alloy steels with a cross-section of 5 mm are hardened in water. Parts with a variable cross-section or a cross-section of less than 5 mm can also be quenched in oil, since the cooling capacity of the oil is sufficient to anneal thin-section parts through and through. Disadvantage: the occurrence of large internal stresses and the formation of cracks.
Hardening in two environments
- this method consists in the fact that a part heated to the required temperature, maintained at this temperature, is transferred to a cooler that provides such a cooling rate that would prevent the decomposition of supercooled austenite in the temperature range of minimum stability of austenite, for example, into water, and then transferred to less an intensely cooling environment in which the hardening actually occurs (2). This method of hardening makes it possible to reduce the level of hardening stresses and prevent the occurrence of hardening defects such as warping. Disadvantage: requires highly qualified workers
Jet hardening
- this method is used when there is no need to harden the part to the same hardness over the entire surface. For such types of parts as a chisel, it is important to obtain a high hardness of the cutting edge while maintaining a tough shank; in this case, the tool, heated to a given temperature, is cooled from the working surface with jets of water, thereby destroying the “steam jacket” and the working surface of the tool is intensively cooled.
Tempering by self-tempering
- this method practically has the same functional purpose as jet quenching, for example, a chisel is heated to a given temperature and only the working part is transferred into a cooling medium, then after removal from the quenching medium, it is held in free air, as a result of which the working part is released due to heating from the non-working, uncooled part. The tempering temperature of the cooled part is controlled visually by the colors of tarnish. Hardening with self-tempering can be carried out using another method; for this, the part (tool) is completely hardened, and then only the non-working part is heated and the working part is tempered due to thermal conductivity.
Step hardening
- this method is a variation of the hardening method in two environments (2). However, from the point of view of part processing, a part with a variable cross-section is more efficient. When cooling to a temperature slightly above the point at which the martensitic transformation begins, it is necessary to equalize the temperature across all sections; to do this, hold it in the first cooler until the temperature is equalized, and then transfer the part to a second cooled environment, in which quenching occurs.
Isothermal hardening
— in contrast to stepwise isothermal hardening, the part is placed in a cooling environment with a temperature slightly higher than the temperature at which the martensitic transformation begins and is kept in this environment until the transformation is completely completed (4). As a result of isothermal hardening, a structure is formed - bainite, which, compared to martensite, has a slightly lower hardness and increased viscosity.
Cold processing of steel.
In hardened steel, especially with a carbon content of more than 0.4-0.5%, there is always residual austenite, which reduces hardness and wear resistance. To reduce the amount of retained austenite in hardened steel, cold treatment is used, which consists of cooling the hardened steel to temperatures below 0. Lowering temperatures to -30 - -70 degrees. For most steels, it causes the transformation of retained austenite into martensite, which increases hardness. But because At the same time, the stresses increase, then the products are cooled slowly and tempering is performed immediately after cold treatment. Cold treatment is used for measuring instruments, springs and parts made of case-hardened high-alloy steels, which retain a lot of austenite after hardening.
Cooling rate
The martensite structure is obtained by rapid cooling of austenite at the moment when the temperature of the steel contributes to the least stability of austenite (about 650-550 degrees).
When moving to the temperature zone in which martensitic transformation occurs (below 240 degrees), slow cooling is applied. As a result, the resulting structural stresses have time to level out while the hardness of the resulting martensite does not decrease.
To carry out successful heat treatment, it is very important to choose the right hardening medium. Often the following can be used as a quenching medium:
- water;
- sodium hydroxide solution (5–10%) or table salt;
- mineral oil.
To harden carbon steel, it is better to use water at a temperature of 18 degrees. Oil is suitable for hardening alloy steel.
Characteristics of steel: hardenability and hardenability
The important characteristics of steel - hardenability and hardenability - should not be confused.
Hardenability
This characteristic indicates the ability of steel to gain hardness after hardening. There are types of steel that are difficult to harden and after the heat treatment process the steel becomes insufficiently hard. They say about such material that it “did not accept hardening.”
The hardness of martensite is related to the degree of distortion of its crystal lattice. A lower carbon content in martensite contributes to less distortion in the crystal lattice, which means the hardness of the steel will be lower. If the steel contains less than 0.3% carbon, then the hardenability of such an alloy is low, and usually such alloys are not hardened.
Hardenability
This characteristic can indicate how deeply the steel has been hardened. When hardening, the surface of a steel part cools faster than the core . This occurs because the surface is in direct contact with the cooling fluid, which removes heat. And the central part of the steel part gives off its heat through the thickness of the metal and the surface, where it is absorbed by the coolant.
Hardenability is affected by the critical hardening rate - the lower it (speed), the deeper the steel is hardened. For example, coarse-grained steel, which has a low critical hardening rate, is annealed deeper than fine-grained steel, which has a high critical hardening rate.
The depth of hardenability depends on the initial structure of the alloy being hardened, the heating temperature and the quenching medium. The hardenability of steel is determined by fracture, microstructure and hardness.
Hardenability of steel
Hardenability is the ability of steel to acquire a martensitic or triple-martensitic structure to a certain depth during quenching. The hardenability of steel depends on the critical cooling rate, which depends on the chemical composition of the steel. So, for example, if the actual cooling rate in the core of a part during hardening is higher than the critical rate for this grade of steel, then the part will have through-hardenability. In this case, the depth of the hardened zone is taken to be the distance from the metal surface to the semi-martensitic structure. Semi-martensitic is a structure that consists of 50% martensite and 50% troostite. The width to the semi-martensitic zone in a cylindrical specimen is called the critical diameter or through-heating section size.
The higher the hardenability of steel, the lower the critical quenching rate, i.e., the higher the stability of supercooled austenite.
The hardenability of steel is determined according to GOST 5657-69 “Steel. Test methods for hardenability.” (the document will open in a new window) GOST describes the so-called end hardening method. The experimental results are expressed graphically in “hardness-distance” coordinates. Those. The graph shows the change in hardness along the cross section after hardening. The hardenability of steel, even within the same grade, can vary significantly. This is due to the fact that hardenability depends on the steel composition, grain size, product geometry, etc. In this regard, the hardenability of steel is characterized not by a curve, but by a hardenability band. It must be taken into account that even rated hardenability strips will not always correspond to the actual hardenability of the product.
Types of steel hardening
There are many ways to harden metal. Their choice is determined by the composition of the steel, the nature of the product, the required hardness and cooling conditions. Stepwise, isothermal and light hardening are often used.
Hardening in one environment
Referring to the graph of cooling curves for various hardening methods, you can see that hardening in one environment corresponds to curve 1. It is easy to perform such hardening. However, it is not suitable for every steel part. Due to the rapid decrease in temperature in steel of variable cross-section, temperature unevenness and high internal stress occur in the temperature range. This can cause the steel part to warp and crack.
Figure No. 2. Cooling curves .
A high carbon content in steel parts can cause volumetric changes in structural stresses, and this, in turn, threatens the appearance of cracks.
Hypereutectoid steels, which have a simple shape, are best quenched in one environment. To harden more complex shapes, hardening in two environments or step hardening is used.
Hardening in two environments (in Figure No. 2 this is curve 2) is used for tools made of high-carbon steel. The method itself consists in the fact that the steel is first cooled in water to 300-400 degrees , after which it is transferred to an oil environment, where it remains until it has completely cooled.
Step hardening
During stepwise hardening (curve 3), the steel part is first placed in a salt bath. The temperature of the bath itself must be higher than the temperature at which the martensitic transformation occurs (240–250 degrees) . After the salt bath, the steel is mixed into oil or air. Using step hardening, you don’t have to worry about the part warping or cracks forming in it.
The disadvantage of this hardening is that it can only be used for carbon steel workpieces with a small cross-section (8–10 mm). Step hardening can be used for parts made of alloy steel with a large cross-section (up to 30 mm).
Isothermal hardening
Isothermal hardening on the graph corresponds to curve 4. Hardening is carried out similarly to step hardening. However, steel is aged longer in a hot bath. This is done in such a way as to cause complete disintegration of the austenite . In the diagram, the shutter speed is shown on the S-shaped line by points a and b. Steel that has undergone isothermal hardening can be cooled at any speed. The cooling medium can be molten salts.
Advantages of isothermal hardening:
- steel is almost impossible to warp;
- no cracks appear;
- viscosity.
Light hardening
To carry out such hardening, a specially equipped furnace equipped with a protective environment is required. In production, to obtain a clean and bright surface of hardened steel, step hardening should be used. After this, the alloy is cooled in molten caustic alkali. Before the hardening process, the steel part is heated in a salt bath of sodium chloride at a temperature 30–50 degrees above point Ac1 (see “Critical point diagram”). The part is cooled in a bath at 180–200 degrees. The cooling medium is a mixture consisting of 75% potassium hydroxide, 25% sodium hydroxide, to which 6–8% water (based on the weight of salt) is added.
Hardening with self-tempering
Used in the production of tool steel. The main idea of hardening is to remove the steel part from the cooling medium until it is completely cooled. The withdrawal occurs at a certain point. A certain amount of heat is retained in the core of the steel part. Subsequent vacations are made at his expense .
After the steel product reaches the required temperature for tempering due to internal heat, the steel is placed in a quenching liquid for final cooling. Figure No. 3—Table of tarnish .
Tempering is controlled by the colors of tarnish (see Figure No. 3), which is formed on a smooth metal surface at 220–330 degrees.
Using self-tempering hardening, sledgehammers, chisels, plumbing hammers and other tools are made, which require high surface hardness while maintaining internal viscosity.
Characteristics of steel
In the context of this topic, steel has two important characteristics.
Hardenability
This characteristic reflects the fact how capable the steel is of becoming hard after undergoing the hardening procedure. There are alloys whose properties practically do not change as a result of this heat treatment, that is, the hardness remains at an insufficient level. They say about such a metal: “does not accept hardening.”
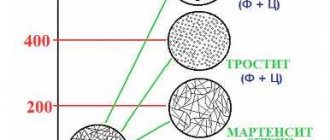
Metallurgy explains the high hardness of carbon-containing martensite by the distortion of its crystalline cells. This factor makes plastic deformation of the material difficult. The hardness index increases with increasing amount of carbon. In numbers it looks like this: the value of this parameter, established using the Rockwell method with the content of the element carbon (C) in steel at the level:
- 0.1%, equal to 30HRC;
- 0.7% is 64HRC.
But a further increase in the amount of carbon in the alloy does not lead to a significant increase in the hardness index. All this is displayed on the graph.
The following designations are used on it:
- pos. “1” – the heating temperature exceeds the Ac3 point;
- pos. “2” – the heating temperature of the product is 770°C, which is higher than only point Ac1;
- pos. “3” is an indicator of martensite hardness.
Typically, alloys with a carbon content of less than 0.3% are not subjected to the hardening procedure due to their low degree of hardening.
Hardenability
This characteristic indicates the depth of hardening of the steel. During this process, the core of the part cools more slowly than its surface. This phenomenon is explained by direct contact of the outer layer with a cooling substance that absorbs thermal energy. The situation is different with the central fragment of the product. Its heat is transferred through the thickness of the metal to the surface area, and there it is absorbed by the same cooling substance.
Hardenability is a characteristic derived from the critical hardening rate. This is understood as the lowest rate of supercooling of all austenite to the martensitic structural transformation. The depth of hardening is inversely proportional to this parameter. That is, the lower the speed of the above process, the deeper the hardening of the metal occurs. This is clearly manifested in alloys with large and small grains. The former are calcined to a greater depth than the latter, since they have a low critical speed.
Cooling methods during hardening
When steel products are rapidly cooled during hardening, there is a risk of large internal stresses occurring, which leads to warping of the material and sometimes cracks. To avoid this, where possible, it is better to cool steel parts in oil. Carbon steel, for which such cooling is not possible, is better cooled in water.
In addition to the cooling environment, the internal stress of steel products is affected by how they are immersed in the cooling environment. Namely:
- products with a thick and thin part in the quenching liquid with the bulky part first;
- If the product has an elongated shape (drills, taps), it must be immersed strictly vertically, otherwise they may warp.
Sometimes it is not necessary to harden the entire part, but only part of it. Then local hardening is applied. The product is not completely heated, but the entire part is immersed in the quenching liquid.
Types of hardening
Quite a lot of methods for hardening metal have been developed today. When choosing a specific one, you need to consider:
- chemical composition of the material;
- design features of the product;
- a given hardness indicator of the final product;
- cooling process conditions.
Quenching in one environment
To better understand the features of the hardening procedure, consider the image below. It shows graphs of cooling lines characteristic of various methods of such heat treatment.
The progress of hardening in one environment is shown by curve “1”. This method can be implemented without any particular difficulties. But it does not apply to all steel products. In particular, problems may arise with parts with variable cross-sections. An accelerated decrease in their temperature indicators leads to:
- formation of internal stresses;
- temperature unevenness.
The combination of these factors usually causes warping and cracking of such products.
When performing this hardening method, cracks can also form in parts made from alloys with a high content of the element carbon. In this case, volumetric transformations of structural stresses cannot be excluded. For hardening in one environment, products with a simple configuration made from hypereutectoid steels are better suited.
Hardening in two environments
This method is represented by curve “2” in the above figure. Hardening in two environments is most often applied to tools made from steels with a high carbon content. This heat treatment method is implemented in 2 stages:
- the product is first immersed in water, where its temperature will enter the range of 300°C≤T≤400°C;
- then the part is moved into an oil-cooling working environment. There the product remains until it cools completely.
Step hardening
The features of stepwise hardening are shown by curve “3”. This method is done like this:
- First, the steel product is placed in a bath of molten salts. Here it is necessary to control that the temperature of the coolant exceeds the martensitic transformation temperature (this range is 240°C≤T≤250°C);
- then the part is cooled in oil or in open space under natural environmental conditions.
With step hardening, the likelihood of warping or cracking of the product is zero. Workpieces with a cross-section of no more than 30 mm, made from steels with alloying additives, as well as products with a cross-section not exceeding 8 - a maximum of 10 millimeters, made of carbon alloys, are subject to such heat treatment.
Isothermal hardening
In the above figure, heat treatment of this type corresponds to curve number 4. The technique for performing it is similar to the previous method. The difference lies in the length of time the alloy is kept in a bath of molten salts. For isothermal hardening, this time interval is longer.
This technological solution ensures comprehensive decomposition of austenite. On the graph, the shutter speed is shown by points “a” and “b” on the S-shaped line. There are no restrictions on the cooling rate of the alloy subjected to isothermal hardening - it can take values from any range. This heat treatment method has another advantage: the metal of the final product acquires toughness.
Light hardening
Carrying out light hardening requires the use of a specially equipped furnace. It must contain a protective environment. To obtain a light surface for the workpiece, which also does not have visible flaws, it is recommended to use step hardening. Upon completion, the steel must be cooled in a molten substance with the following chemical formula: NaOH is a caustic alkali. Before the hardening procedure, the product is heated in equipment called a salt bath filled with sodium chloride. The temperature should exceed the Ac1 point by 20°C-30°C. During cooling, the temperature of the medium is maintained in the range of 180°C-200°C. It includes:
- caustic soda (NaOH) – 25%;
- caustic potassium (KOH) – 75%.
This mixture is diluted with water in an amount of about six to eight percent of the total mass of alkaline components.
Hardening with self-tempering
This method is used in the production of tool steel. The essence of the technology is the removal of a steel product from the cooling environment until it cools completely. After this operation, thermal energy is retained in the thickness of the metal. At her expense, in fact, further vacation is carried out.
But you need to perform subsequent actions related to this procedure while monitoring the temperature of the part. Only when this characteristic reaches the value required for tempering is the product moved to a quenching environment, where it is finally cooled.
Control of the tempering itself is carried out on the basis of tarnish colors. They represent a spectrum of different shades that appear on the surface of the alloy when an oxide film forms on it. This phenomenon occurs when the metal temperature varies in the range 220°С≤Т≤330°С.
Self-tempering hardening is used in the manufacture of hammers for masons and mechanics; chisels of all kinds, from scarpels to cross-mixers; sledgehammers, both sharp- and blunt-nosed. In general, for tools that require high surface hardness without sacrificing toughness.
Defects during hardening of steel
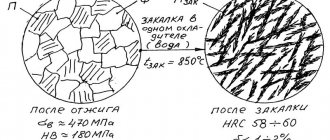
- Insufficient hardness . Occurs if there was a low heating temperature, short holding time at operating temperature, or insufficient cooling rate. The fix is to apply a more energetic environment; anneal and then harden.
- Overheating . Occurs if a steel part is heated to a temperature exceeding the permissible one. When overheated, a coarse-grained structure is formed, which leads to brittleness of the part. Can be corrected: by annealing and hardening at the right temperature.
- Burnout . When a steel part is heated to a high temperature close to the melting point (1200–1300 degrees) in an oxidizing atmosphere. Oxygen penetrates into steel products and oxides form along the grain boundaries. This kind of steel cannot be repaired.
- Oxidation and decarbonization . In this case, scale (oxides) form on the surface of steel parts, and carbon burns out in the surface layers of the steel. This marriage cannot be fixed. To prevent defects, ovens with a protective atmosphere should be used.
- Warping and cracks . They arise due to internal stress. Cracks are an irreparable defect. Warping can be removed by straightening or straightening.
Possible defects during hardening
During the hardening process, some defects may appear in the workpieces. Only the most significant ones are described below.
Insufficient hardness
Insufficient hardness in a product that has undergone a hardening procedure most often appears when:
- the temperature of the heat treatment performed was incorrectly selected;
- the cooling rate was lower than that specified in the flow chart.
For example, during hardening of hypoeutectoid steels, this defect usually occurs due to the retention of ferrite in the structure of the alloy. This phenomenon occurs due to a violation of technology. In this case, the quenching temperature was simply not brought to the value corresponding to point Ac3.
Continuing the conversation about hypoeutectoid alloys, it is necessary to note another possible reason for the insufficient hardness of the material. This is overheating. As a result, martensite is formed, characterized by a coarse-needle structure. This structure not only reduces the hardness of the metal, but also reduces its impact strength. By the way, overheating manifests itself in a similar way in hypereutectoid steels.
Formation of soft spots
The reasons for the formation of soft spots are as follows:
- heterogeneity of the alloy structure;
- during the cooling process, the products came into contact with each other;
- uneven cooling;
- the presence of grease stains on the surface of the parts.
To correct this defect, the product is hardened again. Elimination of structural heterogeneity is carried out by preliminary normalization.
Carbon oxidation and combustion
Decarburization (the so-called burnout of carbon during hardening) and oxidation occur as a result of the interaction of the surface layer of the product with molten salts or furnace gases. The combination of these defects poses a particular danger to cutting tools. Its durability decreases significantly.
Such a defect in heat treatment cannot be corrected. The only thing that can save the situation is a sufficient allowance. Then the defective layers are removed by mechanical processing, and sometimes only grinding is sufficient.
Burnout
Burnout occurs when the heating temperature approaches the melting point of the metal. For this reason, what happens:
- penetration of oxygen into the thickness of the steel, accompanied by the formation of oxides at the grain boundaries;
- melting the material along the grain boundaries. This phenomenon, although rare, does happen.
As a result, the continuity of the alloy is broken, which puts it in the category of irreparable defect. That is, it is unsuitable for use.
Hardening cracks
The reasons for the appearance of hardening cracks are as follows:
- a part in the design of which there were sharp changes in the configuration of sections was subjected to heat treatment. It is in these places that significant internal stresses are formed, causing cracking;
- cooling was carried out extremely quickly;
- heating was carried out unevenly and also unnecessarily accelerated.
Another possible option for the appearance of cracks is that the product was subjected to a tempering procedure with some delay (not directly after hardening), due to which the internal stresses were not leveled in a timely manner.
Warping and deformation
Distortion of the product configuration - warping - causes uneven cooling. The change in volumetric characteristics - deformation - is associated with structural transformations that occur during heat treatment. These defects in the hardened alloy are due to the difference in the specific volumes of the formed structures. In particular, the value of this parameter of pearlite is less than that of martensite. In addition, thermal and structural stresses have different effects on the change in shape of different products.
To prevent the formation of these defects, the cooling procedure must be carried out at a slow rate in the temperature range of martensitic transformation using both isothermal and step hardening methods.