Chemical corrosion of metals is a dangerous phenomenon that can lead to their complete destruction. The process is directly related to the ability of the material to interact with an environment that poses an increased chemical hazard.
Among the important properties of chemical corrosion of metal is the parallel process of oxidation and reduction. Scientists do not note a direct connection with the electric current that could potentially be generated or affect the materials involved in the reaction.
If we consider the root cause of the spread of such a process, we will quickly come to the conclusion that it lies in the thermal instability of metals of different types.
They tend to quickly transition to a stable state under the influence of oxidizing components of the environment, and often this happens completely involuntarily.
The process of oxidation and reduction during chemical corrosion occurs against the background of a decrease in the potential of the system. In this case, it is necessary to take into account the signs of potential changes in order to predict the risk of arbitrarily starting such a process and its intensive occurrence inside the material.
Scientists define the main criterion that stimulates a spontaneous process as an indicator such as the isobaric-isothermal potential G.
When the reaction begins to proceed randomly, it decreases significantly. However, the rate of reduction may vary depending on the type of materials, corrosive environment conditions and a number of other key parameters.
Gas corrosion of metals
Scientific statistics show that gas corrosion of metals occurs most often. When considering chemical deterioration, it is significantly more common than liquid rusting in contact with electrolytes.
An important factor here is high temperature. If the metal is very hot and the gas begins to act on it, destruction occurs.
Since the temperature level must be quite high, and in normal use it is quite difficult to create conditions for such chemical corrosion to occur, the process is often observed in metallurgy.
Because of this, equipment used in stamping, forging, hot rolling and other processes suffers. Without additional protection, the duration of use of such equipment will become significantly shorter.
Contact of metal with oxygen is also dangerous. The reaction formula that starts in this case is as follows: Me + 1/2O2 – MeO.
This reaction has a clear oxidative direction, therefore it is directly related to the indicators of partial oxygen pressure. It is worth paying attention to the fact that the reaction can be equilibrium, shifted towards the formation of an oxide, or proceeding in the opposite direction.
It is very important to understand what gas mixture the metal is in contact with in the area where you are using it. A good understanding of the partial pressure of oxygen in the mixture will give us the temperature range.
It is here that the oxidation process will start, leading to the destruction of the material or a significant deterioration in its quality level.
Factors of the rate of gaseous chemical corrosion
As they studied the characteristics of the chemical corrosion process, scientists were able to identify important factors that affect its speed and other features. These include:
- The temperature of the medium in which the metal is immersed.
- Alloy composition and other features of the metal.
- Features of the gas environment, its composition, predominant elements.
- Duration of contact of the material with a corrosive environment.
- Emerging corrosion product.
As with other types of corrosion damage, the type and characteristics of the oxide film created on the surface are of great importance.
Features of the formation of oxide films during gas corrosion
The entire process of forming an oxide film on a metal surface can be divided into two large stages:
Absorption of oxygen molecules on the surface of a metal product
This occurs in areas that are in direct contact with the atmosphere. The appearance of an ionic bond is noticeable - the oxygen atom takes two electrodes from the metal.
It can be assumed that the formation of a very strong and stable bond during such a reaction is associated with the entry of oxygen into a special field of metal atoms.
When the surface of the material is completely filled with an oxidizing agent, a monomolecular film will be formed. It tends to thicken over time. This reduces further contact with oxygen, but the dangerous corrosion process itself is already running.
Formation of a chemical compound
This phenomenon is typical for a situation in which there is active interaction between metal and gas. Due to the influence of oxidizing components, the alloy begins to actively lose valence electrons. Corrosion products rapidly form and accumulate.
The further course of the process will be largely characterized by the characteristics of the oxide film. So if it has an increased level of protection, the corrosion process itself will slow down.
Types of oxide films
When chemical corrosion occurs under the influence of temperature and gas environment, three types of films can form:
- Thin. It will be impossible to notice them from the outside. They are among the least durable and can be easily erased under mechanical pressure.
- Average. Can be noticed because from the outside the metal changes color slightly.
- Thick. Clearly visible to the naked eye.
In order to prevent dangerous processes of material destruction from occurring, it is important to make the film protective.
Factors in the formation of a protective film
The oxide film can have a pronounced protective effect on the material. But this requires that it meet several important requirements:
- Continuity. On the surface, the film is distributed in an even layer, without pores and areas that are not affected by it.
- Good adhesion to the surface of the material. This is required to keep such a protective barrier in place and prevent deterioration of its properties.
- Chemical inertness. The film will protect the metal only if it enters into chemical reactions with the environment. Otherwise, there is a great danger that the entire protective effect will be reduced to zero.
Since the material will be used for a long time and it is difficult to predict what will affect it, wear resistance and an increased level of hardness are of great importance.
No less important is the fact that the film is not porous and loose. When it is in poor contact with the surface, the risk of destructive processes becomes much higher.
When studying the various properties of oxide films, scientists take a particularly close look at continuity. It is noted that it is influenced by molecular volume. Its indicators must be higher than the atomic volume of the metal.
Continuity is not put in first place when determining the protective properties of the oxide film only for a small group of metals. These include alkaline earth and alkaline.
When carrying out work on protection against chemical corrosion, much attention is paid to the method of measuring thickness. Analysis of characteristics occurs at different stages of formation. Of great importance are the obtained indicators of the rate of metal oxidation and the nature of such a process.
When oxides are formed, experts recommend checking what kind of film they have created on the surface and whether it has the necessary protective properties.
Methods of protection against corrosion
Corrosion control methods include:
- treating the base metal with a protective layer (for example, applying paint);
- use of inhibitors (for example, chromates or arsenites);
- introduction of materials resistant to corrosion processes.
The choice of a specific material depends on the potential efficiency (including technological and financial) of its use.
Modern principles of metal protection are based on the following methods:
- Improving the chemical resistance of materials. Chemically resistant materials (high-polymer plastics, glass, ceramics) have successfully proven themselves.
- Isolation of material from aggressive environment.
- Reducing the aggressiveness of the technological environment. Examples of such actions include neutralization and removal of acidity in corrosive environments, as well as the use of various inhibitors.
- Electrochemical protection (external current application).
The above methods are divided into two groups:
- Chemical resistance enhancement and insulation are applied before the steel structure is put into service.
- Reducing the aggressiveness of the environment and electrochemical protection are used already in the process of using metal products. The use of these two techniques makes it possible to introduce new methods of protection, as a result of which protection is provided by changing operating conditions.
One of the most commonly used methods of protecting metal, galvanic anti-corrosion coating, is not economically viable for large surface areas. The reason is the high costs of the preparatory process.
The leading place among protection methods is occupied by coating metals with paints and varnishes. The popularity of this method of combating corrosion is due to a combination of several factors:
- high protective properties (hydrophobicity, repulsion of liquids, low gas and vapor permeability);
- manufacturability;
- ample opportunities for decorative solutions;
- maintainability;
- economic justification.
At the same time, the use of widely available materials is not without its disadvantages:
- incomplete wetting of the metal surface;
- poor adhesion of the coating to the base metal, which leads to the accumulation of electrolyte under the anti-corrosion coating and, thus, promotes corrosion;
- porosity leading to increased moisture permeability.
And yet, the painted surface protects the metal from corrosive processes even with fragmentary damage to the film, while imperfect galvanic coatings can even accelerate corrosion.
Chemical corrosion in non-electrolyte liquids
Although gas corrosion is considered the most common, metal damage upon contact with various electrolyte liquids should also not be discounted. The greatest danger comes from contact of the material with substances that can conduct electricity.
They are divided into two large groups - organic and inorganic. There are many electrolytes that pose a great danger to metal - from molten sulfur and benzene to liquid bromine, alcohol, kerosene, oil and others.
The purity of the electrolyte is of great importance during the course of a chemical reaction. When it is completely clean, there is no interaction. But as soon as a small amount of impurities gets into the composition, the reaction begins to develop especially rapidly.
Another additional risk factor is the presence of moisture. Then the threat of electrochemical corrosion is also added to the danger of chemical corrosion.
Application of anti-corrosion heat-resistant coatings
This method also involves reducing the rate of the corrosion process, but through special heat-resistant coatings. The technique commonly used is to apply iron-aluminum thermal diffusion layers, which is known as thermochromic plating. Effective protection is also provided by metal-ceramic processing of metal parts and structures. The advantages of such protection against gas corrosion include not only a reliable thermal and mechanical coating, but also the possibility of flexible modification of the physical and chemical properties of the shell. The functional layer can contain both refractory oxides and metal components such as molybdenum and tungsten.
Stages of corrosion in non-electrolyte liquids
If we consider the entire process in more detail and analyze what affects the rate of chemical corrosion, we can distinguish several stages of its occurrence:
- Contact of the oxidizing agent with the surface of the material.
- Starting the process of chemisorption of the reagent on the surface.
- The reaction of the metal and the oxidizing agent, the formation of an oxide film.
Environmental conditions, the composition of the alloy and the electrolyte itself can affect the occurrence of several basic processes. These include such as desorption of oxides with metal and diffusion of oxides into a non-electrolyte. But both processes may also not be observed.
To prevent corrosion from starting in electrolyte liquids, care should be taken to apply special protective compounds to the surface. It is important that throughout the entire period of use of the product they fully maintain their integrity.
Protective atmosphere as a means of combating corrosion
Another technique for protecting metal workpieces and alloys from corrosion as a result of gas oxidation. Protective atmospheres can be formed by environments of argon, nitrogen and carbon. Specific gas mixtures are used for each metal. For example, cast iron is protected with argon or carbon dioxide compounds, while steel interacts well with hydrogen and nitrogen. In the maintenance of main pipelines, this type of protection is used mainly when performing installation welding activities. In continuous operation, electrical protection of gas networks from corrosion is more often used, which is technically performed by semiconductors with cable circuits. This is a type of electrochemical anti-corrosion shell, which includes elements of anode-protective galvanic protection in its structure.
Factors of chemical corrosion
Of great importance when considering the process of chemical corrosion of metals is the identification of factors that influence it. These include:
Temperature
All oxidative processes proceed faster if the temperature increases greatly.
Temperature
Metal products that over a certain period of time begin to cool and heat alternately are in a special risk group. In this case, the protective film suffers greatly. It begins to crack, in places where this happens, the metal comes into contact with the environment, and re-oxidation starts. A new film is also formed against the background of the gradual peeling off of the old one.
Composition of the environment
This applies to both gases and liquid electrolytes. As noted above, even minor fluid contamination can cause the rate of corrosion damage to become significantly higher.
Alloy composition
Various components are added to the metal alloy, which can both slow down and accelerate oxidation. For example, additives such as titanium, copper and cobalt are recognized as strong retarders. Chromium and aluminum have a good effect on reducing the speed of the process.
Surface treatment type
During research, scientists have shown that a smooth surface has a noticeably higher resistance to oxidation, and the process proceeds more slowly. If there are many bumps on the surface of the metal, there are pronounced defects, you should prepare for a faster leakage.
Material structure
The chemical corrosion equation shows that a significant slowdown in the corrosion process is typical for metals with an austenitic structure.
Chemical corrosion. Liquid and gas corrosion
| Item: | Chemistry |
| Kind of work: | Course work |
| Language: | Russian |
| Date added: | 16.04.2019 |
- This type of work is not a scientific work, it is not a finished final qualifying work!
- This type of work is a finished result of processing, structuring and formatting collected information intended for use as a source of material for independent preparation of educational work.
If you have a hard time understanding this topic, write to me on WhatsApp, we’ll look into your topic, agree on a deadline, and I’ll help you!
At this link you can find many ready-made coursework in chemistry:
| Lots of ready-made coursework in chemistry |
Check out these similar threads, they might be useful to you:
| Laws of electrolysis. Application of electrolysis in chemical technology |
| Corrosion of metals. Basic mechanisms and types of corrosion damage |
| Atmospheric corrosion of metals and methods of protection against atmospheric corrosion |
| Electrochemical corrosion of metals and alloys |
Introduction:
Metals and metal alloys are the main structural materials for most sectors of the economy. Their widespread introduction into industrial construction and transport took place at the turn of the 18th-19th centuries. At this time, the first cast-iron bridge appeared, the first ship whose hull was made of steel was launched, and the first railways were created. The beginning of human practical use of iron dates back to the 9th century BC. It was during this period that humanity moved from the Bronze Age to the Iron Age. In the 21st century, high rates of industrial development, intensification of production processes, increases in basic technological parameters (temperature, pressure, concentration of reagents, etc.) place high demands on the reliable operation of technological equipment and building structures. A special place in the complex of measures to ensure uninterrupted operation of equipment is given to its reliable protection against corrosion and the use of high-quality chemically resistant materials in this regard.
Metal corrosion is a spontaneous process, always negative from the point of view of industrial practice. However, there are examples where corrosion plays a positive role and is deliberately provoked, for example, during acid cleaning of metal surfaces. Sometimes the term “metal corrosion” refers not only to the process itself, but also to its result—destruction.
Corrosion is a physical and chemical interaction between a metal and its environment, as a result of which the properties of the metal, environment or technical system that includes them change. A corrosive environment is called corrosive or caustic. As a result of the interaction of metal and an aggressive environment, a chemical compound is formed, called a corrosion product.
Types of corrosion damage
The destruction of metals and alloys can occur as a result of chemical (chemical corrosion, non-electrochemical), electrochemical (electrochemical corrosion), biological (microbiological or microbial corrosion) and radiation (radiation corrosion) effects of the external environment. The ability of metals to withstand the corrosive effects of the external environment is called corrosion resistance.
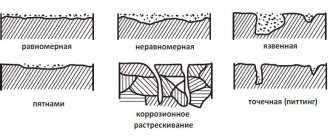
Based on the nature of the corrosion damage, continuous or general, corrosion varies between local and localized. Continuous corrosion is uniform and uneven. Uniform destruction can occur when the metal surface and the properties of the aggressive environment acting on this surface are uniform. In real conditions, corrosion processes are more often found on a non-uniform (heterogeneous) metal surface in a non-uniform aggressive environment. Therefore, in practice they usually deal with uneven corrosion and with various types of localized corrosion.
Corrosion is called uniform if the corrosion destruction front propagates parallel to the plane of the metal, and non-uniform if the corrosion rate in different areas is not the same. An example of uniform corrosion is selective corrosion, which is characteristic of alloys - solid solutions. It consists in the destruction of one of the alloy components.
Thus, when brass corrodes, zinc can be uniformly destroyed, ionized and go into solution, and as a result, the surface layer is enriched in copper.
Localized corrosion has a number of varieties, the most common of which are:
- corrosion in spots in the form of individual shells is the least uneven;
- pitting or pitting corrosion - destruction in the depths of the metal with the formation of pores, even through ones;
- intercrystalline corrosion - destruction of metal along the boundaries of crystals. In this case, external manifestations of the process may not be observed;
- intracrystalline corrosion - destruction of metal along crystallite grains. This type of corrosion occurs when cracking occurs under the influence of external mechanical or internal stresses.
Localized corrosion can be defined as corrosion that occurs on a portion of a metal's surface at a significantly higher rate than the rest of the surface. In particular, pitting (pitting) corrosion is associated with the destruction of the oxide film and often occurs on a completely smooth surface, where the protective oxide film on the metal is subject to only local destruction, but is otherwise resistant to the solution that acts on the metal. Pinpoint damage (pitting) occurs in discontinuities in the oxide film under the most suitable local environmental conditions.
Pitting can occur on the surface of all metals. In practice, this is more common in passive alloys, in which the high-strength film causes corrosion to become localized. One of the reasons for the formation of pits may be the presence of activator ions in an aggressive environment, such as Cl-, Br-, CN-, etc. ions. Pitting corrosion is dangerous because with low corrosion losses the structure can be disabled in a fairly short period of time . Most often, this type of corrosion damage occurs during the operation of products made of aluminum, stainless steel and alloys containing nickel, chromium and other metals prone to passivation.
Intergranular corrosion (ICC) is caused by the fact that grain boundaries, even in pure metal, are more susceptible to corrosion than the grain itself. Metal atoms at grain boundaries have a dense and less ordered packing; impurities are concentrated at the grain boundaries, which further reduces their resistance to aggressive environments. Destruction of grain boundaries is most dangerous when operating metal products under voltage. Under these conditions, so-called intergranular cracking is observed. Aluminum and austenitic stainless steels are more susceptible to this type of damage. To reduce the tendency to MCC, stabilizing elements Ti and Nb are introduced into the steel, which prevents the formation of chromium carbides; however, in welded structures, knife corrosion may occur - rapid destruction in a narrow zone along the welds during oxidation of the environment.
When a metal is subjected to alternating stresses, cracks appear in it and gradually develop. This failure is called a stress fracture. In the presence of an aggressive environment, this process accelerates. This effect is found even in moderately aggressive environments. For example, the fatigue life of aluminum is significantly reduced in the presence of moisture. It is very difficult to protect metals from this type of corrosive attack. Recommended methods of protection (metal coating, anodizing, painting, etc.) Should be used carefully, taking into account the physical and mechanical properties of the protective layer.
Chemical corrosion
Chemical corrosion is a type of corrosive destruction of metal associated with the interaction of the metal and an aggressive environment, in which the metal is simultaneously oxidized and the corrosive environment is restored. Chemical corrosion is not associated with the formation or exposure to electric current.
The driving force (main cause) of chemical corrosion is the thermodynamic instability of metals. They can spontaneously transform into a more stable state as a result of the process: Metal + Oxidizing component of the medium = Reaction product.
In this case, the thermodynamic potential of the system decreases.
The sign of the change in thermodynamic potential can be used to determine the possibility of spontaneous chemical corrosion. The criterion is usually the isobaric-isothermal potential G. When a chemical process occurs spontaneously, a decrease in the isobaric-isothermal potential is observed.
Therefore, if:
- D GT <0, then the process of chemical corrosion is possible;
- D GT> 0, then the process of chemical corrosion is impossible;
- D GT = 0, then the system is in equilibrium.
Chemical corrosion includes:
- Corrosion in non-electrolyte liquids;
- Gas corrosion - corrosion when metal comes into contact with dry gases at high temperatures (for example, corrosion of metals in internal combustion engines, combustion chambers, jet nozzles under the influence of gaseous products of fuel combustion).
Chemical corrosion in non-electrolyte liquids
Non-electrolyte liquids are liquid media that are not conductors of electricity. These include: organic (benzene, phenol, chloroform, alcohols, kerosene, oil, gasoline); inorganic origin (liquid bromine, molten sulfur, etc.). Pure non-electrolytes do not react with metals, but with the addition of even a small amount of impurities, the reaction process accelerates sharply.
Corrosion in liquid non-electrolytes comes down to a chemical reaction with the substance:
- Me + Br2 (liquid) > MeBr2;
- Me + S (melt) > MeS.
Sulfur is a chemical corrosive agent in liquid fuels. Sulfur in the molten state reacts with almost all metals, significantly destroying tin, lead, copper, low-carbon steels and titanium, and also slightly destroying aluminum. The heavier the distillation fraction in the series gasoline - kerosene - fuel oil, the higher the sulfur content. Different sulfur compounds react differently. Hydrogen sulfide forms insoluble compounds with many metals - sulfides.
Elemental sulfur also forms sulfides when reacting with metals. Organic compounds containing sulfur (R-SH mercaptans) react with metals to form organometallic derivatives - mercaptides.
The presence of water increases the corrosivity of crude oils containing thioalcohols and hydrogen sulfide. Straight distilled gasoline in the absence of water has virtually no corrosive effect on ferrous alloys. Cracked gasolines, when interacting with metals, become resinous, and the acidity of the environment increases, which promotes corrosion.
If, in addition, the temperature rises, oxygen will be dissolved in the liquid - chemical corrosion will intensify. The presence of moisture in non-electrolyte liquids ensures intense corrosion through an electrochemical mechanism. For example, when carbon tetrachloride contains traces of water, the corrosion rate of steel increases sharply: CCl4 + H2O = CCl3OH + HCl which is associated with the formation of aggressive products during hydrolysis. Even anhydrous chlorinated organic solvents are destroyed by aluminum.
Chemical corrosion in non-electrolyte liquids is divided into several stages:
- approach of the oxidizing agent to the metal surface;
- chemisorption of the reagent on the surface;
- reaction of an oxidizing agent with a metal (formation of an oxide film);
- desorption of oxides by metal (may be absent);
- diffusion of oxides into a non-electrolyte (may be absent).
To protect structures from chemical corrosion in non-electrolyte liquids, coatings that are stable in this environment are applied to its surface.
Gas corrosion
The most common case of chemical corrosion is the oxidation of metals by various gases. This occurs at elevated temperatures, when moisture condensation on the metal surface is impossible. Rocket engine nozzles, furnace fittings, internal combustion engine parts, and gas turbine blades are subject to gas corrosion. Metals are subject to gas corrosion during heat treatment.
Gaseous corrosive agents include O2, CO2, SO2, H2O, H2S, Cl2. For the reaction of metal oxidation with oxygen 2Metv + O2 (g) = 2MeRef.
They do not have the same aggressiveness towards metals. The oxidation rate increases in the order H2O>CO2>O2>SO2 (at 900 °C for iron, cobalt, nickel). Moreover, in the atmosphere of these gases, the corrosion rate decreases to the order of Fe> Co> Ni.
The equations for these oxidation processes are:
- Fe + H2O > FeO + H2
- Fe + CO2 > FeO + CO
- Fe + H2O + SO2 > FeSO3 + H2
- 2Fe + O2 > 2FeO
- 3Fe + SO2> 2FeO + FeS.
The mechanism of gas corrosion is due to the occurrence of two conjugate reactions at the interface between the solid and gas phases. One of them is the oxidation of the metal, the other is the reduction of the gaseous oxidizer, and these processes are not separated in space. The products of the oxidation reaction accumulate there. During the formation of corrosion products, metal atoms and ions, on the one hand, and oxygen atoms or ions, on the other, diffuse through a gradually thickening film of corrosion products. As a result, corresponding compounds are formed on the metal surface, for example, oxides and sulfides.
Steel, cast iron, iron, which interact with oxygen, are subject to gas corrosion. They lose strength and hardness, especially at temperatures above 300 ° C. In this case, products are formed according to the reaction: Fe + O2 > FeO + Fe3O4 + Fe2O3.
The resulting mixture of products is called dross. At the same time, decarbonization of metals occurs: Fe3C + O2> Fe + CO2.
Decarburization also occurs in a hydrogen atmosphere: Fe3C + 2H2 > 3Fe + CH4^.
Thermodynamics of chemical corrosion of metals
As mentioned above, the main cause of chemical corrosion of metals is their thermodynamic instability in various environments under given external conditions, as a result of which the metals spontaneously transform into a more stable oxidized (ionic) state. This process is accompanied by a decrease in the thermodynamic potential. The Gibbs free energy DG can be used as a criterion for the equilibrium and spontaneity of metal corrosion processes occurring at constant temperatures and pressures. Under these conditions, the process of chemical corrosion is possible if DG <0; the corrosion process is impossible if DG> 0; the system is in equilibrium if DG = 0.
A judgment about the thermodynamic feasibility or impossibility of a process can be made directly based on a comparison of the partial pressure of oxygen in an aggressive gas environment and the dissociation pressure of the resulting oxide PMeO. For the metal oxidation reaction (5), the change in the Gibbs free energy can be determined by the isotherm equation.
POO2, PO2 are equilibrium and nonequilibrium oxygen pressure, respectively. Under normal atmospheric conditions, PO2 is the partial pressure of oxygen and is ~0.21 atm. The equilibrium pressure of oxygen P0O2 is equal to the dissociation pressure (elasticity) of the resulting oxide PMeO. Since for most technically important metals the elasticity of dissociation of their oxides POO2 to melting temperatures is several orders of magnitude less than 0.21 atm, the DG value is negative and the metal oxidation reaction proceeds spontaneously. Only the noble metals - gold, platinum, palladium, iridium - have a POO2 > 0.21 atm under normal atmospheric conditions. and are resistant to oxidation. All other metals are unstable.
Kinetics of chemical corrosion
Chemical corrosion kinetics studies the patterns of film growth on metals. These laws are expressed by mathematical relationships of the form h = f (f), where h is the thickness of the corrosion product film; f - time. The true corrosion rate of the metal will be determined by the derivative dh/df.
Gas corrosion processes are multi-stage heterogeneous processes occurring at the metal-gas interface.
When a metal interacts with gaseous substances, which is accompanied by the formation of a porous oxide film on the metal surface, the following successive stages can be distinguished:
- transportation of gases to the interface;
- gas adsorption on the metal surface;
- chemical interaction;
- removal of corrosion products.
Porous growth film. Porous loose films do not protect the metal from corrosion, since the oxidizing gas easily penetrates through the pores onto its surface and enters into a chemical interaction. The reaction rate in this case does not depend on the thickness of the resulting film.
The equation of the straight line is called the linear law of film growth. In it, a = h at φ = 0. According to this law, alkali and alkaline earth metals are oxidized.
Continuous film growth. Continuous, dense films of corrosion products on metals are protective and prevent the penetration of reagents - metal and oxidizing agent - towards each other.
When a continuous (protective) film is formed on the surface of the metal, the process can be divided into the following separate stages:
- transition of metal in the form of ions and electrons from the metal phase to the oxide;
- movement of metal ions and electrons in the oxide layer;
- transport of oxygen to the surface of the oxide film;
- ionization of adsorbed oxygen;
- movement of oxygen ions in the oxide layer;
- chemical reaction to form an oxide.
The growth of such films is accompanied by a slowdown in the process, a decrease in the corrosion rate as the film thickens. The growth of thin oxide films at low temperatures and in the first stages of metal oxidation at high temperatures is accompanied by strong self-development. The logarithmic law corresponds to this case.
The protective properties of the film are determined by a number of factors, the continuity of which is a necessary but not sufficient condition. The oxide film must have good adhesion to the base and metal and be sufficiently elastic and durable. The thermal expansion coefficients of the metal and the oxide film must be close in value to prevent cracking of the films. The oxide film must be very resistant to corrosion. The Pilling-Bedworth ratio is used to characterize the continuity of oxide films. If VOKC/VMe is 1, then the film can be continuous. In this respect, V0KC represents the volume of the metal-oxygen (or other oxidizing agent) compound; VMe is the volume of metal used to form V0KC.
Mechanism of chemical corrosion
The nature of the reagents, their physical state, including the degree of dispersion and the degree of defectiveness of solid phases, the pressure of the gaseous medium and temperature play a significant role in the mechanism of the process. When a clean metal surface is oxidized in an air atmosphere, the main cause of all phenomena in the boundary layer should be considered oxygen adsorption.
Since oxygen is a strong oxidizing agent, this adsorption, even at low temperatures, is of a chemical nature, that is, it is accompanied by the breaking of bonds in the oxygen molecule and the ionization of the resulting atoms:
- O2 (g) = O2 (ads);
- O2 (ad) = 2O (ad);
- O (ad) + s (Me) = O- (ad).
As a result, a layer of negatively charged ions appears on the metal surface adjacent to the cationic layer. An electronic potential gradient appears inside the boundary layer, reaching 107 V/Ohm.
Mott and Cabrera were the first to explain the appearance of oxide films on metals at low temperatures, when there is practically no diffusion, under the influence of a strong electric field. Thickening the film to several tens of nanometers leads to a sharp decrease in the electric potential gradient, and the field energy is insufficient for the growth of the oxide layer. The oxidation process stops. Further oxidation is only possible at higher temperatures, when thermal diffusion becomes decisive.
The oxide film, which initially appears on the surface of the metal, has the peculiarity that its structure is subject to a certain orienting effect of the surface atoms of the substrate. The phenomenon of orientational and dimensional correspondence of the newly formed crystalline phase to the surface of the old one is called epitaxy. Thus, P.D. Dankov and his colleagues discovered that during the oxidation of iron, orientational correspondence occurs with the formation of FeO, Fe3O4 and g-Fe2O3 on b-Fe; Fe3O4 on FeO, g - Fe2O3 on Fe3O4. Epitaxial correspondence determines the ease of formation of new crystal structures not only from a purely geometric side, but also from the energy side, i.e. corresponds to the principle of lowest energy consumption when forming a new lattice. As the oxide layer thickens, the effect of orientation forces weakens and the oxide tends to adopt a structure that is resistant to these conditions. As a rule, the chemical composition of the atomic layers of scale is heterogeneous: the metal content decreases towards the outer surface, and the oxygen content increases. Thus, on iron and steel with long-term oxidation above 850 ° K, macroscopic layers of different phase composition are in the sequence: Fe> FeO> Fe3O4> r-Fe2O3> b-Fe2O3. As the temperature increases, diffusion is the determining process for the formation of oxide layers. Diffusion in solids, in which there is long-range order in the arrangement of particles, has some distinctive features.
This can happen through the following mechanisms:
- exchange of ions or atoms in places;
- movement along internodes;
- movement on vacancies.
The first mechanism is associated with great energetic difficulties and is therefore unlikely. The second and third mechanisms are more probable, since they are caused by the presence of real defects in the crystals (Frenkel and Schottky defects).
The influence of external and internal factors on the rate of chemical corrosion
External factors are related to the composition of the aggressive environment and corrosion conditions (temperature, pressure, medium speed, etc.). Internal factors are factors related to the nature of the metal, the composition and structure of the alloy, the nature of surface treatment, etc. Temperature greatly affects the rate of chemical (gas) corrosion of metals. With increasing temperature, oxidation processes occur much faster. The temperature dependence of the corrosion rate is quantitatively expressed by the Arrhenius equation: where K is the chemical reaction rate constant for kinetic process control or the diffusion coefficient for diffusion control; A is the pre-exponential factor; E is the activation energy of a chemical reaction or diffusion; R is the universal gas constant; T is temperature on the Kelvin scale. Temperature fluctuations, especially alternating heating and cooling, increase the rate of metal oxidation as the protective oxide film develops cracks due to thermal stress and can peel off. Composition of the gas environment. The gas environment has a significant effect on the corrosion rate, and this effect is specific. For example, nickel is relatively stable in O2, H2O, CO2 environments and is highly corrosive in SO2 environments. Copper is actively oxidized by atmospheric oxygen, but it is stable in an SO2 atmosphere, while chromium has high heat resistance in all of the above environments. The composition of the gas environment has a great influence on the rate of oxidation of iron and steel. O2, sulfur compounds and water vapor are particularly affected.
The speed of movement of the gas medium has a noticeable effect on gas corrosion only at the very initial stages of oxidation. This is equivalent to an increase in the partial pressure of the oxidizer. Very high speeds can destroy protective films and accelerate gas corrosion.
Alloy composition. Having a direct impact on the protective properties of product films, some alloy components lead to a decrease in the oxidation rate, others, on the contrary, accelerate oxidation, while others have virtually no effect on the oxidation rate.
As for the most important and widespread structural material - iron-based alloys - the following can be noted: S, P, Mn - have virtually no effect on the rate of chemical corrosion; Be, Ti, Co, Ni - significantly slow down the oxidation of iron, which is associated with an increase in the protective properties of the resulting scale; Cr, A1 and Si - significantly slow down the oxidation of steel due to the formation of highly protective oxide films; they are widely used for alloying steel in order to increase its heat resistance; V, Mo, W - can accelerate the oxidation of steel at high temperatures, due to the fusibility and volatility of the resulting oxides. Metal construction. An increase in the defectiveness of the metal structure leads to an acceleration of oxidation.
Metal deformation somewhat accelerates oxidation in the initial stages due to an increase in the energy of the metal and the effect on the structure of the primary oxide film.
The nature of the metal surface treatment mainly affects the rate of the initial stages of oxidation. The more thoroughly the metal surface is treated, the lower the rate of its oxidation.
Protection of metals from chemical (gas) corrosion
The main ways to reduce the rate of gas corrosion of metals are heat-resistant alloying and the application of protective coatings. Heat-resistant alloying.
In the theory of heat-resistant alloying, there are two mechanisms for the formation of protective films:
- formation of a protective layer from an alloying element. According to this theory, a protective oxide of the alloying element forms on the surface of the alloy, which slows down the diffusion of reactants and the oxidation of the base metal. This theory agrees well with a number of cases of oxidation of alloys and allows, based on knowledge of some properties of elements and their oxides, to qualitatively assess the possibility of increasing heat resistance during alloying;
- formation of highly protective double oxides. According to this theory, V.I. formulated most fully. Arkharov, the alloying element can form double oxides with the base metal - such as spinel, for example, MeMe2O4, which have increased protective properties. The main alloying elements for iron are Cr, Ni, Si, A1. They promote the formation of double oxides of a spinel structure with close packing and small lattice parameters, for example: Fe2Cr2O4, FeAl2O4, NiFe2O, NiCr2O4. The considered theories of heat-resistant alloying do not exclude, but complement each other and allow a more rational approach to the development of formulations of new heat-resistant alloys.
Conclusion:
Corrosion causes billions of dollars in losses every year, and solving this problem is a major challenge. The main damage caused by corrosion is not the loss of metal per se, but the enormous cost of the products destroyed by corrosion. This is why the annual losses from this in industrialized countries are so high. The true losses from this cannot be determined by estimating only the direct losses, which include the cost of the destroyed structure, the cost of replacing equipment and the cost of corrosion protection measures. Indirect damages cause even more damage. This includes equipment downtime when replacing corroded parts and assemblies, product leakage, and disruption of technological processes.
Ideal corrosion protection is 80% ensured by proper surface preparation, and only 20% by the quality of the paints and varnishes used and the method of their application.
Let's sum it up
In this article, we looked at the types of chemical corrosion and how it differs from electrochemical corrosion. Provided that a number of requirements are met, the risk of metal destruction can be significantly reduced. These include:
- Control of the formation of a protective oxide film.
- Excluding contact of certain materials with aggressive environments.
- Use of additional protective coatings.
- Monitoring the composition of a gas mixture or electrolyte.
- The use of metals with alloy components that slow down the reaction.
Compliance with these requirements will significantly increase the duration of use of your metal product.
Return to articles Share article
Organosilicate coatings
For high-quality protection against corrosion, it is recommended to use metals with a high level of hydrophobicity and impermeability in water, gas and steam environments. These materials include organosilicates.
Chemical corrosion practically does not apply to organosilicate materials. The reasons for this lie in the increased chemical stability of such compositions, their resistance to light, hydrophobic properties and low water absorption. Organosilicates are also resistant to low temperatures, have good adhesive properties and wear resistance.
The problems of metal destruction due to corrosion do not disappear, despite the development of technologies to combat them. The reason is the constant increase in metal production volumes and increasingly difficult operating conditions for products made from them. It is impossible to completely solve the problem at this stage, so the efforts of scientists are focused on finding ways to slow down corrosion processes.