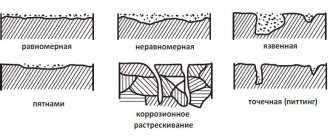
Intercrystalline corrosion of metal is a common process that can lead to complete failure of the product and loss of all operational characteristics.
Research shows that almost all types of steel are susceptible to corrosion. Somewhere it develops faster, somewhere slower. The nature of the lesion, the rate of spread, and the level of damage differ.
If we look at intercrystalline corrosion in more detail, we can see that it is an electrochemical process. During its occurrence, the destruction of the metal occurs along the boundaries of crystalline grains.
The main catalyst is external environmental pressure.
Experts note the high danger of such a process. The reason lies in its hidden flow. Rust appears inside the metal and does not make itself felt from the outside for a long time.
By the time the process begins to break through to the surface, there is a serious danger that all performance characteristics will be completely lost and the material will become too brittle.
Sometimes a structure can collapse suddenly.
The following types of materials are most prone to intercrystalline corrosion:
- aluminum alloys;
- steels containing more than 13% chromium, copper, nickel, alloyed with molybdenum;
- austenitic and stainless steels.
The process can occur in other materials, but those listed are most often affected.
Features of the appearance and course of intergranular corrosion
Most experts define intergranular corrosion as an electrochemical reaction. Its occurrence becomes possible if high oxidative values are achieved.
Upon closer inspection, you will notice that anodes and cathodes are created along the grain boundary. Rapid dissolution of the anode is also observed. Against this background, spaces of heterogeneity develop.
Thus, internal destruction begins, which will gradually begin to come to the surface.
Among the main causes of intergranular corrosion:
- Strong rise in external temperature.
- Constant contact with aggressive environments.
- Features of the alloy composition.
Materials that contain a large number of alloying additives fall into the risk zone. In this case, individual sections of the metal can transition from a passive to an active state. Various types of internal processes become the stimulus.
The main parameter that affects the rate of rusting is an indicator of the potential of the metal. Research shows that even when analyzing a single part subject to corrosion, it is possible to detect areas with different rates of corrosion.
The highest speed is shown by the corrosion process at a potential of 1.15-1.25V.
Another important concept is embrittlement. It means a gradual narrowing of the zone of influence in austenitic materials. This type of defect most often begins to appear in places where severe overheating begins to occur during the welding process.
When a part is exposed to elevated temperatures, rapid grain growth begins. Against this background, rapid evolution of hydrogen also occurs.
Modern industry uses various proven methods to prevent the process of corrosion damage. An example is the use of tempering technology at temperatures up to 150 degrees for one to two hours.
Another method of protection is to prevent splashes of hot metal during the melting and welding process. Pressure builds up at the welding site, which should also be avoided.
Treatment with organosilicon varnish or primer works well. It is important to properly prepare the surface for applying the composition.
Mechanism of intercrystalline corrosion
Intergranular (transgranular) corrosion
is a type of corrosion that occurs at grain boundaries without deep corrosion of the metal matrix.
It occurs under two conditions
:
Metallographic condition refers to irregular material or structure that is established at grain boundaries for various reasons. This condition is caused by lattice modifiers (crystalline defects) or the presence of accumulated heterogeneous phases at the grain boundaries during the crystallization process (impurities) or during a thermal process that caused their precipitation at the grain boundaries. Environmental conditions determine different effects on grain boundaries. These differential effects may result from the action of selective dissolution of the metal into a state of higher reactivity or the formation of galvanic regions in which the anodic regions are grain boundaries while the cathodic regions are the matrix of the metal spacer.
Intergranular corrosion can occur even at high temperatures through the penetration of elements in the gaseous state (for example, nickel in the presence of sulfur and sulfides) to the grain boundaries. Intergranular corrosion involves many metals such as aluminum, stainless steel, nickel alloys, etc. The nature of this form of corrosion is very insidious as it affects the microstructural level without visible corrosion products on the outer surface of the metal. Thus, this form of corrosion leads to depletion of the bond between grains with deterioration of mechanical properties and crack propagation between the boundaries of the destroyed grain, that is, when the applied force is of high intensity. In severe cases, this form of metal corrosion leads to grinding (Fig. 1) with catastrophic and critical consequences.
As for stainless steels, they are susceptible to intergranular corrosion when subjected to heat treatment, such as "hypersensitivity" to this form of corrosion. Sensitization of stainless steels occurs after any heat treatment between 400 and 900°C (eg TIG and MIG welding). At this temperature, the carbon present in the alloy tends to form carbides with chromium, which is also present in the alloy. These carbides are deposited at grain boundaries, i.e. at points of increased activity, and subtract elemental chromium, necessary to obtain a passive layer and increase the corrosion resistance of steel.
Figure 2 shows the trend of elemental chromium relative to the cross-section of the two most closely sensitized grains. Near the ordinate, the chromium content is very high due to the intense formazine of chromium carbides at the grain boundaries. Immediately after this, the chromium content drops rapidly to such levels as to be less than the limit beyond which the steel is passive when simply exposed to air. This reduction in elemental chromium will result in intergranular corrosion if the steel comes into contact with a suitable corrosive environment.
How does intergranular corrosion manifest itself in austenitic stainless steels?
Austenitic steels are often used in industry. This is a special format of material that is associated with the use of austenite - a solution with a two percent carbon content.
There are a large number of austenitic alloys that contain a large percentage of nickel and chromium. The concentration is at the level of 15% and 7%, respectively.
Another condition for classifying an alloy as an austenitic category is the level of alloying components not exceeding 55%.
Upon closer examination of the structure of austenitic metals, it is possible to discover that corrosion processes have a minimal impact on such raw materials.
Austenitic steels, due to their good corrosion protection, are actively used in various fields of industry, including chemical and oil production.
The main requirement in order to prevent the spread of corrosion in alloys of this type is not to exceed the content of carbon and sulfur in the formulation.
The quality and different connections of such components can also negatively affect the quality. Such steels have good fusibility characteristics and are easy to process.
Mechanism of intercrystalline corrosion
Today it is known that corrosion can form on any metal product. Some of them can resist the harmful effects of corrosion for a longer period than others.
There are different types of corrosion processes. One of them is intercrystalline corrosion - this is an electrochemical process of destruction of metal along the boundaries of crystalline grains under the influence of the environment. The destruction of metal under the influence of intercrystalline corrosion is recognized as one of the most dangerous, since destruction processes begin inside the material, where they are not visible to the human eye. While such destruction manifests itself on the outer surface, the entire internal part may lose its operational characteristics (strength, stability, deformability) and lead to premature, and most importantly, unexpected destruction of the entire structure, which will entail an emergency situation that can cause harm to workers close to people.
The intercrystalline lattice often damages various aluminum alloys; steels containing more than 13% chromium; copper, nickel, alloyed with molybdenum; austenitic and stainless steels, etc.
Main aspects of protecting stainless steels from corrosion
The name stainless steel itself is given because of the high level of protection against the gradual development of corrosion. The material has pronounced passivation properties.
The metal can become passive because chromium is added to it. The level of its content has always seriously influenced the level of anti-corrosion resistance.
Also, how well a material resists corrosion is influenced by many characteristics, including the degree of carbon concentration. If the volume of carbon in the alloy increases, the quality of rust protection becomes less.
Another parameter that begins to influence corrosion resistance is the structure of the alloy. If the material becomes heterogeneous, the level of chromium in it increases, and the likelihood of corrosion damage becomes greater over time.
Also among the important indicators of corrosion resistance is a good level of protection when in contact with oxidizing environments.
316 stainless steel
Stainless steel
In all grades of stainless steel, the main components responsible for the corrosion resistance and ductility of the metal are chromium and nickel. The addition of >10% chromium makes the steel stainless by creating a layer on the surface containing large amounts of chromium oxide. This layer is formed as a result of the reaction of chromium contained in the alloy with oxygen from the atmospheric air. It gives the steel a property that makes it stainless. The addition of nickel provides good ductility and improved forming and welding properties.
However, not all bar stock is created equal. Nickel and chromium content of Swagelok 316/316L stainless steel tube fittings and instrument valves exceeds the minimum requirements of ASTM standards for rods and forgings.
It should be taken into account that although stainless steel of different grades is not subject to complete corrosion, local corrosion may occur on it.
To combat:
continuous corrosion; hydrogen embrittlement; intergranular corrosion;
Material matters
The risk of stress corrosion cracking increases at high chloride concentrations, temperatures and tensile stresses. All grades of stainless steel are susceptible to stress corrosion cracking. We have tested Swagelok pressure tubing fittings for SCC resistance and found excellent results.
Swagelok 316 stainless steel tube fittings and instrument valves exceed the minimum requirements of ASTM standards.
Basic methods of protecting stainless steel from intergranular corrosion
In order to increase the level of corrosion protection of intergranular steel, several standard methods can be used. These include:
- Reducing carbon levels. If there is less of it in the alloy, the likelihood of intergranular corrosion becoming much less. Most often, this remedy is used if other options are not suitable. The reason is the high cost.
- Hardening. Processing the workpiece under high temperature is necessary if the finished part will not be used under high heat.
- The use of additional special additives. When tantalum or titanium is introduced into the alloy, the possibility of increasing the concentration of carbon, which could negatively affect the degree of corrosion intensity, is eliminated.
Factors that increase the risk of corrosion
These factors include:
- Carbon content:
the higher the content, the greater the risk of this form of corrosion; - The temperature
for the austenitic range is 650-700°C; - Time sensitization:
maximum degree of sensitization occurs after approximately 10,000 minutes; - Grain size:
Coarse grains will be more susceptible to sensitization than fine grains; - Cold processing:
increases the area of deposition of chromium carbides; - Addition of Alloying Elements:
Addition of titanium or niobium in percentages promotes the formation of titanium or niobium carbide (as it is most closely associated with carbon), which stabilizes the grain boundary and leaves the chromium present in the alloy intact.
Methods for testing the resistance of alloys to intergranular corrosion
To understand that the material is not subject to intergranular fracture, it is worth paying attention to special tests. The inspection is carried out in full compliance with the requirements specified in GOST 603289.
The test standard is carried out with different types of steels. These include alloys such as:
- Austenitic;
- Ferritic;
- Austenitic-ferritic;
- Iron-nickel.
Test methods that check the level of resistance to intergranular corrosion may vary. These include conducting accelerated tests, as well as placing them in a solution with fluorine and copper.
The test uses samples that fully reflect the characteristics of the material and help understand how different corrosion catalysts will affect the raw material.
Return to articles Share article
Stainless steel corrosion - how to protect yourself from it?
- Reducing the carbon concentration in alloys, since carbon is an element that promotes the development of intercrystalline corrosion. This method of protection is used extremely rarely, since steel itself is expensive, and carbon helps reduce this price without losing performance characteristics.
- For steels that will work under normal conditions and will not be exposed to high temperatures, it is advisable to use hardening.
- Introduction of special additives such as titanium and tantalum. Such substances stabilize the amount of carbon and prevent it from reacting, thereby protecting against the formation of rust.
Attention! Our company produces anti-corrosion protection for metal structures of any complexity: pipelines, tanks, silos.
Source of the article: https://math-nttt.ru/obrabotka-metalla/mezhkristallitnaya-korroziya-nerzhaveyushchih-stalej-2.html