Often, when you pick up an object shiny with metal, you are surprised at its light weight and realize that it is plastic or wood with a metal coating. Chemical metallization helps to obtain a mirror or matte nickel, chrome, copper or aluminum coating on ordinary ferrous metal or a non-metallic surface. Technology available not only in production, but also at home.
Process technology
Electrolytes are substances that can release ions – charged particles – under the influence of an electric current. This is what the principle of galvanization is based on. In our case, chromic anhydride will be used as an electrolyte. Released particles that will be deposited on the workpiece, forming a film - chromium molecules.
To chrome a part at home, it must be immersed in a bath of solution and connected to the negative wire. The positive anode is immersed in the electrolyte. Under the influence of current, the molecules in the electrolyte will begin to move. Positively charged to the minus (cathode), negative - to the plus. Moreover, some of the molecules form a film, and some will penetrate into the top layer, as a result of which chromium is firmly fixed on the surface. This makes galvanizing significantly different from conventional painting.
In a similar way, not only chrome plating is carried out, but also nickel plating, coating of products with copper and zinc. The processing principle will be the same in any case. The thickness of the spraying will depend on the current strength, heating temperature, processing time, and type of metal.
It is also possible to carry out chemical chrome plating at home. No special equipment is required here. The formation of a metal film on the surface in this case occurs due to chemical reactions in which sodium hypophosphite serves as a reagent. But such a coating is less durable - it is used only for decorative purposes.
This is interesting: KGShP (crank hot stamping press): characteristics and features
Galvanization
The galvanic method is the coating of cast iron, steel, brass or copper structures with a layer of chromium. But not only metal products can be chrome-plated by galvanization. This method can also be used for chrome plating of plastic and wooden products. But in these cases the process will be expensive and technologically complex. To firmly hold the chrome coating on the surface of parts, even metal products require another preliminary coating. For this purpose, nickel, brass or copper are used.
Galvanization requires the creation of a galvanizing plant. In addition, you need a DC source and a set of reagents. This set consists of chromium anhydride, sulfuric acid, soda ash and sodium hydroxide.
It should be noted that when working with this method, it is required that there are no changes in current strength. You also need to constantly monitor the level of salt concentration in the electrolyte and strictly observe the temperature regime for quite a long period (from 5 to 8 hours). Fulfilling all of the above conditions in home workshops is not an easy task. It is for this reason that we will not describe the galvanization process in detail in this review.
Required Equipment
Electroplating (chrome plating) at home is possible if you have the following type of equipment:
- power supply: it should show 1A at the output and be equipped with a voltage regulator; for small volumes of work, a current rectifier is sufficient; the wiring cross-section depends on the size of the workpiece (minimum 6.25 mm);
- wires: the positive one will be immersed in the electrolyte, the negative one, with an alligator holder, will be located at the end towards the workpiece;
- anodes made of tin, lead or antimony alloys;
- containers of suitable size made of chemically resistant, non-conductive material; the ideal option is a plastic bath; for chrome plating of small parts, a glass jar is sufficient;
- a wooden box with thermal insulation made of glass or mineral wool in which the container will be placed; Ordinary sand can also be used as insulation;
- sealed lid: it can be made from a piece of plywood or wooden boards;
- heating element, the power of which is sufficient to heat the liquid in the selected container to a temperature of 60-80°C;
- contact thermometer or thermostat;
- a hollow mold for pouring electrolyte with a tap or brush at the end; to make it, a bundle of copper wire is used, secured and tied with lead wire.
What you need for chrome plating
The premises have already been mentioned. Now about equipment and materials.
Constant voltage source
Metallization technology involves the use of various electrolytes and containers commensurate with the dimensions of the sample being processed. Therefore, if we are not just talking about chrome plating a single part, then it should be possible to adjust the current strength. The simplest homemade power supply used at home is a transformer + rectifier + rheostat.
Electrical circuit elements
This mainly concerns the cross-section of wires. Practice shows that 3 “squares” is quite enough for home chrome plating of parts. It is also necessary to prepare the clamps with which they will be fastened to the electrodes. There is a crocodile type on sale. The cost is cheap, and the ease of working with them is maximum.
How to ensure constant temperature in the bath? There are two ways. The first and easiest is to use a thermometer and adjust the current value manually. The second one is more difficult to implement, since you will have to install an automatic element - a thermostat. Its inclusion in the circuit will eliminate the need to control the process.
The approximate time for chrome plating is known, so constant presence near the tank is not necessary. But for this you will have to assemble a small electronic circuit. For those who do not understand this, this option is unacceptable.
Bath
Its material must be neutral with respect to liquids, especially aggressive ones. Therefore, glass is best. But this applies mainly to small-sized parts that can fit, say, in a standard 3-liter jar. As an option - containers from industrial batteries with thick walls. Aquarium enthusiasts often raise fry in such aquariums. But getting these vessels is quite difficult.
"External" capacity
You need to assume that the selected glass vessel will be installed in it, which will be lined with insulation on the outside. What can you use? For example, a barrel, a can, a box or a box (even a wooden one).
Thermal insulation material
The choice is large, depending on the size of the containers - sawdust, mineral wool, sand, fiberglass. The goal is to achieve the “thermos” effect. Therefore, it is necessary to make a lid that should completely cover the “outer” tank. If this is a box, then a fragment cut from FC, OSV or something similar will do. It is only necessary to provide waterproofing of the cover from the inside if it is made of a material that includes wood. What to consider? Whatever the cover is made of, it should not be conductive!
A heating element
To make your task easier, you can purchase it. The range of heating elements is quite large, so choosing according to configuration, size and power is not a problem.
Electrodes
They can be plate or rod (for example, made of brass, copper). As a “minus” at home, a clamp is usually used to hold the sample being processed.
bracket
This “detail” cannot be avoided. The workpiece, which is placed in the electrolyte for metallization, must be in a suspended state. Otherwise, the part that will be adjacent to the bottom of the vessel will remain unprocessed. The design of the bracket and the method of its fixation are chosen independently, depending on the work conditions.
Drying cabinet
At the last stage of metallization, the part must be subjected to heat treatment. Otherwise, you will not achieve the characteristic (and desired) shine.
Procedure for preparing electrolyte
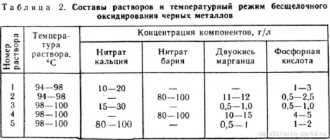
There are quite a lot of methods, as well as the reagents used. For chrome plating at home, the most common electrolyte option is based on sulfuric acid and chromic anhydride. Component ratio: 2.5 g H2SO4 + 250 g CrO3 per 1 liter of clean water.
- First, the bath is filled. Water (distilled is the best) is poured in approximately ½ full. Its optimal temperature is 60 – 65 ºС. Under such conditions, the dissolution of the chromium compound will occur faster.
- Load CrO3 and mix thoroughly until all grains disappear.
- If necessary, add a certain amount of water (to the required volume), then add sulfuric acid.
After mixing the resulting solution, it is subjected to “processing”. It consists in passing a direct current through it. Its strength is calculated from the ratio: 1 liter - 6.5 A. Visually, the readiness of the electrolyte can be determined by its shade. It should turn dark brown in color. After this, the solution settles in a dark and cool place for at least 24 hours.
When choosing a capacity by volume, you need to focus on the power of the power supply. Accordingly, it should be selected based on the dimensions of the parts to be metallized. This is another reason why chrome plating of large samples at home is impractical and difficult.
Course of action
To do chrome plating at home using a special galvanic brush, you can use the following procedure:
- The fibers are tightly wrapped with lead wire.
- The wrapped bristles are placed in a transparent cylinder (preferably made of plexiglass). The top of the container is covered with a lid equipped with a filling hole and a metal contact. One of the ends of the lead winding made is soldered to this contact.
- Small through holes need to be made in the foam plastic membrane located above the bristles.
- A 12-watt transformer is used as a rectifier. The plus is supplied to the contact, which is attached to the cover, the minus is fixed on the element being processed.
- The electrolyte contained in the cylinder penetrates the bristles through holes made in the membrane.
When using any method, a compressor or a good vacuum cleaner will be useful to remove dust.
Electrolyte preparation
To calculate the volume of electrolyte ingredients, you should adhere to the following ratios, measured in grams, per liter of pure water:
- Chromium anhydride - 250 g;
- Sulfuric acid - 2.5 g.
A glass vessel is filled halfway with settled and boiled water, the temperature of which should be approximately 60 degrees. Chromic anhydride is then placed into the container. The solution is stirred until the substance dissolves, after which sulfuric acid is carefully poured into it.
Then the composition must be kept under current for three and a half hours. If the calculations are made correctly, the electrolyte will turn dark brown. Having de-energized the composition, it must be left for one day in some cool and dark place.
Part preparation
Before you do chrome plating yourself at home, you need to prepare it. Rust, varnish, dirt and paint must first be removed from surfaces to be treated. Once the cleaning is complete, you can begin degreasing.
Experts point out that it is undesirable to use gasoline and white spirit for this purpose, because these compounds will negatively affect processing. It is better to use a special mixture based on caustic soda, soda ash and silicate glue
The solution must be heated to 90 degrees Celsius and the part should be immersed in it for about half an hour. If the element has a complex configuration, then the holding time can be increased.
Chrome plating in stages
Due to the high toxicity of free chromium, all work must be carried out in a non-residential area equipped with ventilation. It is strictly prohibited to flush electroplating waste down the drain. For them, you should prepare a special chemical-resistant container made of glass or plastic.
- The anode is immersed in the bath. The negative cathode is connected to the transformer.
- The workpiece is suspended at a certain distance from the walls of the container. She shouldn't touch them.
- The current is connected.
- The color of the coating will depend not only on the type of electrolyte, but also on the temperature of its heating. If it is below 35°C, the film will be matte. You can achieve shine by raising the temperature to 35-55°C. Milky chrome plating is obtained by heating above 55°C.
- The exposure time depends on the thickness of the future coating. The average duration is 2-3 minutes.
- Do-it-yourself chrome plating of plastic at home is not much different from electroplating other types of materials. The part is placed in a container with electrolyte and connected to the negative wire.
- Since chromium cannot adhere to all types of metals (only brass, nickel and copper), coating steel products requires the creation of a substrate layer.
- The process begins with a “push” of current. In the first 20-60 seconds, its density should be 2 times higher than the working one. This allows for better treatment of recessed areas. Then the current density is set to the recommended value and the part is processed for another 1-1.5 minutes.
- The temperature must be maintained at the same level throughout.
- The last stage is double rinsing with water. After the first, the electrolyte is neutralized using alkali (regular baking soda).
- The finished product should be polished using paste.
So, chrome plating parts yourself is a real process. Of course, obtaining a wear-resistant coating at home will be problematic - this will require currents of up to 100 A. But a thin, durable film of chromium is quite capable of protecting parts and components from moisture and corrosion. Electroplating also allows you to create original items for interior decoration.
This is interesting: Metal turning - all about turning technology
Removing poor-quality coating
There are two ways to remove low-quality chrome. The first is chemical dissolution, carried out in a 50% solution of sulfuric acid. The products are placed in a container with sulfuric acid and kept until the coating is completely dissolved. The second is the anodic dissolution method, carried out in a galvanic bath. Products are immersed in a bath of 20% caustic soda solution and connected as an anode; steel sheets or parts are used as a cathode. The process takes place at a temperature of 70-80C and an anodic current density of 20-25 A/dm2 until chromium is completely dissolved. Before repeated chrome plating, the products are heated for 1.5 hours at a temperature of 150-200C to remove hydrogen.
Preparing the part for metallization
While the electrolyte is settling, it's time to do this.
Removing contaminants
In order for chrome plating to be of high quality and inexpensive, the layer covering the part must be uniform and thin. This can be achieved if the surface is cleaned down to the very base. How to remove foreign fractions and degrease is up to you to decide. For samples with smooth edges, as a rule, “sandpaper” is sufficient. In other cases, you will have to think about how and with what to remove dirt and rust.
Degreasing
This is the second preparatory stage. Limiting yourself only to traditional means - gasoline, white spirit, solvent “666” or something similar - means you will not achieve high-quality metallization. Chrome plating on such a surface will not last long.
Additional processing is carried out in a solution that is specially prepared for these purposes. There are many recipes, but the most popular for metallization at home is the following:
150 (caustic soda) + 50 (soda ash) + 5 (silicate glue).
*Based on g/l of water.
The pre-treated workpiece is immersed in this solution, which must be brought to a temperature of 85 (±5) ºС. The exposure time depends on the relief of the part and the degree of its residual contamination (from ⅓ to 1.5 hours).
Features of the chrome plating process
The “installation” diagram with the voltage switching polarity is shown in the figure (see above).
- The current is supplied after the sample is immersed in the electrolyte with some delay. Why? High-quality metallization will occur only if the temperatures of the part and the solution are equal. So you'll have to wait a little. This time can be reduced if the sample is preheated to 40 - 50 degrees. For example, in front of the reflector.
- Recommended current is within 55 ºС.
- After the entire part is covered with a layer of chrome, without “bald spots” (determined visually), it must be dried in an electric cabinet (at least 2.5 - 3 hours).
Before finishing the article, the author with great regret must add a couple of “flies in the ointment”.
Those who are not afraid of the listed difficulties need to take into account a number of other problems that they will have to face. Some - for example, ensuring high quality polishing, maintaining the absolute accuracy of the recipe, the difficulty of determining the optimal dimensions and shape of the electrodes, maintaining a constant current regime - will seem insignificant against the background of just one.
Where can I get anhydride and “pure” acid? The first one is on sale, but only for legal entities, with the preparation of the relevant documents. Store-bought H2SO4, which is purchased for the purpose of preparing electrolyte for batteries, is not suitable. It is not “clean” enough, so it will not provide high-quality chrome plating.
The article turned out to be quite complete. Whether or not to engage in metallization at home is up to you, dear reader, to decide.
Advantage
The purposes of metallization are varied, in most cases it is to impart or increase certain qualities:
- resistance to corrosion processes;
- resistance to mechanical damage;
- wear resistance;
- decorativeness.
The quality of the film depends on the composition of the metal:
the cheapest zinc coating increases anti-corrosion qualities, is actively used in construction to protect embedded parts, steel sheets are coated with zinc before coating with plastics and profiling;
- chromium increases hardness, imparts heat resistance, and makes products attractive in appearance;
- aluminum coating protects parts of equipment operating at elevated temperatures (up to 900°C);
- coating with copper or tin gives a noble appearance even to plastic objects;
- silver produces a mirror-like shine.
When carrying out work, the main condition for obtaining results is adherence to technology.
The essence of technology
Chemical metallization technology can be used to achieve a variety of purposes, most of which are associated with changing the decorative qualities of the surface. In addition, this processing method allows you to hide the main defects of metal or other material: microscopic cracks and pores, other structural defects. In some cases, technology is used to restore coverage.
The essence of the technology is to apply a thin layer of metal. The specifics of the substance application process depend on the specific technology, of which there are quite a few.
Metallization allows you to impart certain performance qualities to parts. Among the achieved characteristics of the processed product, we note the following:
- Hardness increases. Metal is more durable than plastic. By covering the surface of plastic or wooden products with it, you can protect the base from mechanical stress.
- Decorative properties increase. The metallic glossy finish looks very attractive.
- The wear-resistant qualities of the surface are improved. Metallization is often carried out in order to reduce friction between contacting parts.
Parts after chemical metallization
Parts that have high hardness and wear resistance are used in a wide variety of fields. However, high performance can only be ensured if all recommendations are followed.
Classic metallization technology has the following features:
- Several reagents are applied, which react to form a surface layer with certain performance properties. There are many different methods for transferring reagents to workpieces, each with its own characteristics, advantages and disadvantages.
- As a result of metallization, a protective layer is formed on the substrate. In this case, a reliable connection is formed between the coating and the substrate, which is maintained over a long period.
- The resulting coating can be of a wide variety of shades. If necessary, you can create a transition from one color to another without a clear boundary. In some cases, when it is necessary to obtain a surface with special decorative qualities, a dye is added during metallization.
Chemical metallization of different colors
The chemical treatment in question can be carried out in a home workshop, despite the fact that metallization is considered a complex technological process. As a rule, at home the workpiece is subjected to catalytic chrome plating. Due to this, the coating becomes attractive and gains protection from moisture.
Chemical metallization of metal is also in demand because it can be used at home. The work is carried out according to the following algorithm of actions:
- The part is cleaned of contaminants. There should be no layer between the surface layer and the base, as this will significantly reduce the performance characteristics.
- Degreasing is carried out. It is carried out using a special alkaline solution or a special detergent that can remove organic contamination from the surface.
- The degreased surface is additionally washed with clean water. In a similar way, previously used compounds can be removed from the surface during degreasing.
- Surface areas that should not be exposed to the chemical are treated with lead. The tests carried out indicate that lead does not react to the influence of an electrolytic solution.
- Wiring is connected to the solution bath to supply electricity, after which the part is lowered into the prepared reagents.
- After the required period has passed, the product is removed from the solution, dried, and then cooled. If the coating is of high quality, then it is polished.
To carry out the process under consideration at home, you can purchase a special mini-installation designed for chemical metallization, which runs on a small compressor.
The process in question should only be carried out in compliance with the following recommendations:
- Before immersing the part in the bath and applying electricity, you need to check that all contacts are connected securely and can withstand the load.
- The room in which the work in question will be carried out must be ventilated. For this purpose, a ventilation system is installed. This recommendation is due to the fact that during the metal plating process gases are released that can negatively affect vision and breathing.
Parts subjected to chemical metallization
To comply with metallization technology, you must have sufficient experience. You should not expect that when carrying out a complex operation of transferring one material to another for the first time, you will get a result that can be achieved using special industrial equipment.
How to determine steel grade
There are simply a huge number of different steel options, each grade is characterized by its own specific features. If the manufacturer has not carried out markings, then you can find out the characteristics of the metal only by independently conducting various tests. We'll talk about this in more detail later.
How to determine steel grade
Methods for determining steel grade
A fairly common question is how to determine the grade of steel. There are several common methods:
- The first involves removing chips from the surface, for which a chisel can be used. At high carbon concentrations it will be short and brittle. A decrease in the indicator causes an increase in plasticity. However, it is not possible to accurately determine the brand using this method.
- The second method involves hardening the product, after which it is necessary to make cuts. If the material is simply sawed before and after hardening, then it contains a small amount of carbon. Due to the increase in carbon concentration after treatment, the surface becomes too hard.
- Determining the steel grade by spark is based on a visual inspection of the sparks that are formed when processing the surface with a grinding wheel. With an increase in the size of the sparks and their number, the hardness index increases, which depends directly on the carbon concentration. Such a test does not give an accurate result, since the main characteristics of the flying chips depend on the force of pressing and some other points. You can find tables that decipher the qualities of a material based on chips.
Spark test methodDevice for determining steel grade
You can also determine the brand by the color of the sparks produced. For this purpose, special tables were compiled. The test can be carried out at home only if the lighting is correct.
However, it is impossible to accurately identify the material in this way.
The option with alloying elements can also be identified by other performance characteristics, for example, resistance to high humidity or strong magnetism.
In the CIS, the applied designation standards are characterized by the fact that they can be used to indicate the main elements. When considering the issue of decoding the brand, we note the following points:
- The abbreviation “St” is often used. In other cases, no abbreviations are used at all, only numbers.
- In most cases, the first number indicates the carbon concentration. The following can be used to indicate the amount of alloying components.
- The composition may include alloying components that significantly change the properties of the material. An example is the inclusion of chromium, which increases resistance to high humidity.
Classification of steels by purpose
Labeling is deciphered using tables that indicate the designation of the chemical element.
Marking of steels according to international and CIS standards
In order to decipher a brand, you can use a variety of standards. Some alloys are designated by certain symbols that indicate the purpose of the metal.
An example is the following points:
- The letter “Ш” is used to designate metals that are used to make bearings. They are characterized by increased wear resistance.
- High-quality alloyed workpieces are designated by the letter “L”. Often the symbol is indicated at the end.
- “T” is used to designate heat-strengthened rolled products.
- High corrosion resistance of the workpiece is determined by the letter “K”.
- If copper is included in the composition, then the symbol “D” is used when indicating the brand.
- Instrumental can be identified by the letter “U”. They are often used in the manufacture of various tools that are characterized by high wear resistance.
- The symbol “P” is indicated to designate alloys that contain tungsten. Such a substance significantly increases the heat resistance of the structure.
By deciphering the brand, you can determine which chemical elements are included in the alloy. The numbers in most cases indicate the concentration, symbols, type of alloy and specific chemical elements.
European steel marking system Carbon steel grades according to GOST and ISO international standards
In conclusion, we note that there is simply a huge number of products on sale; in many cases, the brand is affixed by the manufacturer. It is almost impossible to independently determine the composition without the use of special equipment.
, please select a piece of text and press Ctrl+Enter.
Chemical metallization
Chemical metallization is the formation of a thin film of metal on the surface being treated under the influence of various chemical reagents. This method can be used to obtain coatings with zinc (zinc plating), chromium (chrome plating), aluminum (alitizing) and others. Using this technology, it is possible to obtain an even layer of metal on materials with different types of surface: smooth - glass, porcelain, polished stone, or porous: wood, plastic, gypsum.
Silver metallization
Performing chemical metallization at home is quite possible, but requires careful preparation.
Workplace and equipment
As a result of the chemical reaction, a gas is released that negatively affects the mucous membranes of the respiratory tract, so the process must be carried out in a room with forced ventilation or in an open space.
Equipment you will need:
- enamel bath;
- measuring cups with a capacity of 1 l and 250 ml;
- 3 bottles of 100 ml;
- disposable syringes for 5, 20, 50 ml3;
- disposable glasses of 50 ml;
- kitchen electronic scales.
Don’t forget to get rubber gloves, a respirator, sponges, and a set of protective clothing, since when working with concentrated hydrochloric acid, caution is required, otherwise burns are inevitable.
Reagents
Depending on the material of the workpiece and the type of coating, reagents are purchased. For chemical metallization with silver, you will need the following reagents:
- hydrochloric acid;
- silver nitrate;
- tin chloride;
- sodium hydroxide;
- ammonia;
- glucose;
- formalin;
- distilled water.
Preparation of solutions for:
- surface activation - stannous chloride, hydrochloric acid, distilled water;
- recovery - glucose, formaldehyde, distilled water;
- silvering - silver nitrate, sodium hydroxide, ammonia, distilled water.
Surface preparation
the surface is prepared in several stages. Porous and painted products are sanded, the old paint layer is removed, the surface is cleaned of dust, washed and degreased. You can degrease with white spirit, acetone or a solution of sodium hydroxide in water t= +40...+60°C. The surfaces are wiped with a sponge containing a degreasing compound, then washed with distilled water with another sponge. The prepared surface must be completely wetted with water, without dry spots - in these places defects will be inevitable.
Coating the product with an activating composition
The object to be treated is poured with tin chloride evenly over the entire surface for 1 minute, then washed with distilled water for 3 minutes.
Metallization
To obtain a uniform metal film, a solution of a reducing agent and silver plating is sprayed onto the product simultaneously and in equal volumes. Since the resulting mirror film is very thin and not durable, it can be strengthened with a protective varnish - transparent or tinted.
The described method resembles the painting process. There is another, more complex way of performing work - electrochemical metallization.
Chrome plating at home
I would like to share my experience of decorative chrome plating in real home conditions. This can be done using this starting set of reagents for creating a mirror in chrome, gold, copper and any other shade on any surface. The kit contains reagents that are not available in regular stores. These are silver nitrate, stannous chloride, sodium hydroxide, glucose and sodium thiosulfate. Everything else can be bought in regular stores and is available to everyone, this is acetic acid 70% (in grocery stores), ammonia or ammonia 10% (in a pharmacy), distilled water (in auto parts). You will also need scales up to 300 g. (radio parts stores), measuring cups (also suitable for food products), disposable cups, spoons, syringes and hand sprayers. To obtain a chrome-look mirror, you need to go through 4 stages: preparing solutions degreasing the surface activating the surface metallization I’ll start with the recipe for decorative chrome plating, which is so carefully guarded in the depths of their workshops by people who are fluent in the art of chrome plating.
Chemical reagents used
Chemical metallization technology involves the use of various substances that bind together to form a protective coating after undergoing a chemical reaction. Using an activator and reagents for chemical metallization, you can do without special equipment, but the method is not suitable for large parts.
To carry out the processing in question you will need:
- The reducing agent is the main component. Chemical metallization reagents should be stored according to the recommendations provided by the manufacturers.
- The activator is also an important reagent that determines the performance of the surface. Chemical metallization reagents have labels that indicate the name of the metal. Let's take gold, mel and chrome as an example.
- The primer is applied to the surface to ensure the most favorable processing conditions. It significantly increases the adhesion of the applied metal.
- The varnish protects the applied coating from chemical and mechanical influence.
- In order to give the surface a certain color, special toners are used. The specific shade is indicated on the toner packaging.
Reagents for chemical metallization
It is worth considering that when doing the work yourself, it is quite difficult to ensure high quality surfaces. In some cases, you have to use special cleaning compounds.
Considering the disadvantages of chemical metallization, we note that when carrying out this procedure, harmful chemicals are used, work with which must be carried out in strict compliance with safety precautions. This technology is quite simple to implement and resembles the method of coating a surface with a paint and varnish substance.
Materials and equipment used
Chemical metallization, as mentioned above, can be done with your own hands and in a home workshop. Moreover, products that are small in size and simple in shape are processed using this method even without the use of special equipment. If you have such equipment at your disposal, then you can chemically apply a layer of metal even to large parts of complex configurations.
When performing this procedure yourself, you should be extremely careful, as this involves using chemicals that are hazardous to health. If you properly prepare the equipment and materials for performing chemical metallization, then you can obtain coatings on various products with your own hands at home, the quality of which is practically no different from those formed at the factory.
Reagents for chemical metallization
The kit for chemical metallization must contain reagents that have the properties of an activator and a reducer. To perform this procedure, you will also need a primer, which is applied to the surface to be treated, and a varnish that protects the finished coating from the negative influence of external factors. To apply the final varnish coating, you should choose a material that has high hardness and wear resistance.
To color the applied metal layer in the desired color, you can use a special color toner. The primer mentioned above is necessary in order to improve the adhesion of the applied metal layer to the material from which the product being processed is made. The result of do-it-yourself chemical metallization may not always be of high quality. However, the applied coating can be removed using special removing solutions.
The chemical metallization unit is designed for coating any hard surface