What is the most fragile metal?
Osmium is a grey-bluish, hard but brittle metal with a very high specific gravity.
Interesting materials:
Is it possible to do nail extensions without a lamp? Is it possible to have ear surgery? Is it possible to get a piercing at 14 years old without parental permission? Is it possible to have a wake 9 days later? Is it possible to have a third caesarean section? Is it possible to keep a money tree in the house? Is it possible to add photos to Instagram from a computer? Is it possible to take a parrot out of its cage? Can you trust Polygraph? Is it possible to go to Turkey without a tour operator?
What is a magnet and how does it work?
A magnet is a body that has its own magnetic field. There are several types of magnets:
- Permanent - products that, after a single magnetization, retain this property. Magnets are divided into several subtypes depending on strength and other parameters.
- Temporary - they function on the principle of permanent ones, but only when they are located in a strong magnetic field. For example, products made from so-called soft iron (nails, paper clips, etc.).
- Electromagnets are wires tightly wound around a frame. As a rule, such a device is equipped with an iron core. It works only if electric current passes through the wire.
A permanent magnet is the most familiar and widespread. For its manufacture, the following combinations of materials are most often used:
- neodymium-iron-boron;
- alnico or UNDC alloy (iron, aluminum, nickel, cobalt);
- samarium cobalt;
- ferrites (compounds of iron oxides and other ferrimagnetic metals).
Magnetism
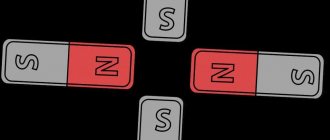
Any magnet has a south and north pole.
Like poles repel, and opposite poles attract. Interesting fact : magnets are often made in the shape of a horseshoe. This is done to ensure that the poles are located as close to each other as possible. This creates a strong magnetic field that can attract larger pieces of metal.
Scientific point of view
To determine which metals are not magnetic, you need to find out how all metals in general can relate to magnets and a magnetic field. With respect to the applied magnetic field, all substances are divided into diamagnetic, paramagnetic and ferromagnetic.
Each atom consists of a positively charged nucleus and negatively charged electrons. They move continuously, which creates a magnetic field. The magnetic fields of electrons in one atom can enhance or cancel each other, depending on the direction of their movement. Moreover, the following can be compensated:
- Magnetic moments caused by the movement of electrons relative to the nucleus are orbital.
- Magnetic moments caused by the rotation of electrons around their axis are spin moments.
If all magnetic moments are equal to zero, the substance is classified as diamagnetic. If only spin moments are compensated - to paramagnets. If the fields are not compensated, use ferromagnets.
Paramagnets and ferromagnets
Let's consider the option when each atom of a substance has its own magnetic field. These fields are multidirectional and compensate each other. If you place a magnet next to such a substance, the fields will be oriented in one direction. The substance will have a magnetic field, a positive and a negative pole. Then the substance will be attracted to the magnet and can itself become magnetized, that is, it will attract other metal objects. For example, you can magnetize steel clips at home. Each one will have a negative and a positive pole, and you can even hang a whole chain of paper clips on a magnet. Such substances are called paramagnetic.
Ferromagnets are a small group of substances that are attracted to magnets and are easily magnetized even in a weak field.
Search magnet for gold and silver and its properties
Typically, powerful magnets are designed to find precious metals. A search magnet reacts to gold and silver quite strongly, and although it is difficult to find them in their pure form, its power is enough to pick up jewelry and coins from the ground. The main goal of all search engines is treasures, expensive coins, and sometimes just ferrous metal.
The article will describe the structure of the magnet and the basic principle of operation. He will also figure out what exactly can be found with its help and how to find expensive alloys. It will be explained in detail what ferromagnets, paramagnets and diamagnetic materials are. In addition, valuable tips and recommendations will be given that will greatly simplify the search for valuable items.
general information
Copper (cuprum) is a metal that has a golden-reddish color and is characterized by high thermal and electrical conductivity. Another distinctive quality of the element is its high ductility. Nuggets are found less and less often in nature; they are most often mined from ore.
When determining the authenticity and purity of a sample, you can turn to an expert, but determining a chemical element in a laboratory is quite expensive. Therefore, you need to focus on several home methods.
Properties of aluminum
The main physical properties of aluminum and aluminum alloys that are useful for application:
These properties of aluminum are presented below in the tables [1]. They can only be considered as a basis for comparison of alloys and their states and should not be used for engineering calculations.
They are not guaranteed values, since in most cases they are average values for products with different sizes, shapes and manufacturing methods.
Therefore, they may not be completely representative of products of all sizes and shapes.
Nominal densities of popular aluminum alloys are presented for the annealed state (O). Differences in density are due to the fact that the alloys have different alloying elements and in different quantities: silicon and magnesium are lighter than aluminum (2.33 and 1.74 g/cm3), and iron, manganese, copper and zinc are heavier (7.87 ; 7.40, 8.96 and 7.13 g/cm3).
For information on the influence of the physical properties of aluminum and, in particular, its density, on the structural characteristics of aluminum alloys, see here.
Aluminum as a chemical element
- Aluminum is the third most abundant - after oxygen and silicon - among about 90 chemical elements found in the earth's crust.
- Among the metal elements, it is the first.
- This metal has many useful properties, physical, mechanical, technological - thanks to which it is widely used in all spheres of human activity.
- Aluminum is a malleable metal that has a silvery-white color and is easily processed by most metal forming methods: rolling, drawing, extrusion (pressing), forging.
- Its density - specific gravity - is about 2.70 grams per cubic centimeter.
- Pure aluminum melts at a temperature of 660 degrees Celsius.
- Aluminum has relatively high thermal and electrical conductivity coefficients.
- In the presence of oxygen, it is always covered with a thin, invisible film of oxide. This film is largely impermeable and has fairly high protective properties. Therefore, aluminum generally exhibits stability and long service life under normal atmospheric conditions.
Combination of properties of aluminum and its alloys
Aluminum and its alloys have unique combinations of physical and other properties. This has made aluminum one of the most versatile, cost-effective and attractive structural and consumer materials.
Aluminum is used in a very wide range - from soft, highly flexible packaging foil to the most demanding space projects.
Aluminum is rightfully second after steel among numerous structural materials.
Low density
Aluminum is one of the lightest industrial structural materials. The density of aluminum is approximately three times lower than that of steel or copper. This physical property provides it with high specific strength - strength per unit mass.
Figure 16 – Automotive aluminum profiles for absorbing impact energy in an accident
Fireproof properties
Aluminum parts do not form sparks when hitting each other, as well as other non-ferrous metals. This physical property is used for increased fire safety measures on structures, for example, on offshore oil rigs.
At the same time, with an increase in temperature above 100 degrees Celsius, the strength of aluminum alloys decreases significantly (Figure 17).
Figure 17 – Tensile strength of aluminum alloy 2014-T6 at different test temperatures [3]
Technological properties
The ease with which aluminum can be processed into any form - manufacturability - is one of its most important advantages. Very often it can successfully compete with cheaper materials that are much more difficult to process:
- This metal can be cast by any method known to foundry metallurgists.
- It can be rolled to any thickness, down to foil, which is thinner than a sheet of paper.
- Aluminum sheets can be stamped, drawn, upset and formed using all known metal forming methods.
- Aluminum can be forged using all forging methods
- Aluminum wire, which is drawn from a round bar, can then be woven into electrical cables of any size and type.
- There are almost no restrictions on the shape of the profiles into which this metal is produced by extrusion (pressing).
Figure 18.1 – Sand casting of aluminum
Figure 18.2 – Continuous casting and rolling of aluminum strip [5]
Figure 18.3 – Upsetting operation in the manufacture of aluminum cans [4]
Figure 18.4 – Aluminum forging operation
Figure 18.5 – Cold drawing of aluminum
Figure 18.6 – Pressing (extrusion) of aluminum
Sources:
- Aluminum and Aluminum Alloys. – ASM International, 1993.
- A. Sverdlin Properties of Pure Aluminum // Handbook of Aluminum, Vol. 1/ed. G. E. Totten, D. S. MacKenzie, 2003
- TALAT 1501
- TALAT 3710
- Technical Brochures
- Application of aluminum
| 31.10.2017 16:13 The material in question presents ten ways to distinguish aluminum from stainless steel. Some of them are very easy to use at home, without having absolutely any tools, equipment or chemicals. This will allow you to quickly and accurately determine the value of a particular item (product) made of aluminum or stainless steel. MagnetUnfortunately, it is not always possible to reliably distinguish these two metals from each other using a magnet. The fact is that any brand of aluminum, one way or another, does not stick to the magnet. But not all stainless steel has the same property. If the product under study is magnetic, then it is definitely not aluminum. The sample may refer to stainless steels that contain sufficient amounts of nickel. If copper or chromium predominates in stainless steel, then it will not react in any way to a magnet. MarkingAs a rule, some stainless steel products have appropriate markings that allow you to accurately identify the item being examined. In this case, everything is quite simple. Inscriptions like “STAINLESS” and other similar ones are a clear sign that this is definitely not aluminum. Plain paperOne of the easiest ways to determine the difference between aluminum and stainless steel. For the experiment you will need a sheet of plain paper. It must be white paper. The one used for printer printing is suitable. The denser it is, the better for the business. The essence of the experiment is as follows. First, you need to clean the edge of the product under study from dirt, grease, oils and other deposits. Next, you need to move this place along a sheet of white paper. The pressing force should be as strong as possible. It is very easy to draw conclusions. Stainless steel on a white sheet will not leave any marks, while aluminum will show thin stripes of gray color. |
Distribution of paramagnets and diamagnets in the periodic table of Mendeleev elements
The magnetic properties of simple substances change periodically with increasing atomic number of the element.
Substances that are not attracted to magnets (diamagnets) are located mainly in short periods - 1, 2, 3. Which metals are not magnetic? These are lithium and beryllium, and sodium, magnesium and aluminum are already classified as paramagnetic.
Substances that are attracted to magnets (paramagnets) are located mainly in the long periods of the Mendeleev periodic system - 4, 5, 6, 7.
However, the last 8 elements in each long period are also diamagnetic.
In addition, three elements are distinguished - carbon, oxygen and tin, the magnetic properties of which are different for different allotropic modifications.
In addition, there are 25 more chemical elements whose magnetic properties could not be established due to their radioactivity and rapid decay or the complexity of synthesis.
The magnetic properties of lanthanides and actinides (all of which are metals) change irregularly. Among them there are para- and diamagnetic materials.
There are special magnetically ordered substances - chromium, manganese, iron, cobalt, nickel, the properties of which change irregularly.
Non-magnetic stainless steels
Stainless steels that are not magnetic include chromium-nickel and chromium-manganese-nickel. They are usually divided into several groups.
Austenitic
The most popular brand of such stainless steels, which occupy a leading place among non-magnetic steel alloys, is 08Х18Н10 (international analogue according to AISI 304 classification). Steels of this type, which also include 08Х18Н10, 08Х18Н10Т, 12Х18Н10Т, 10Х17Н13М2Т, are actively used in the production of equipment for the food industry; kitchenware and cutlery; plumbing equipment; containers for food liquids; refrigeration equipment elements; containers for food products; medical supplies, etc.
Composition and application of austenitic steels
The great advantages of such stainless steel, which does not have magnetic properties, are its high corrosion resistance, demonstrated in many aggressive environments, and manufacturability.
Austenitic-ferritic
Steels of this group, the most popular grades of which are 08Х22Н6Т, 08Х21Н6М2Т and 12Х21Н5Т, are distinguished by a high chromium content and a low nickel content. To give such a stainless steel the required characteristics (an optimal combination of high strength and good ductility, resistance to intergranular corrosion and stress-corrosion cracking), elements such as copper, molybdenum, titanium or niobium are introduced into its chemical composition.
Chemical composition of some industrial grades of austenitic-ferritic steels (click to enlarge)
In addition to the above, stainless steels that are not magnetic include alloys with an austenitic-martensitic and austenitic-carbide structure.
Hard magnetic materials
Hard magnetic materials are used to make permanent magnets. These materials must meet the following requirements:
- have a large residual induction;
- have a high maximum magnetic energy;
- have stable magnetic properties.
The cheapest material for permanent magnets is carbon steel (0.4 - 1.7% carbon, the rest is iron). Magnets made of carbon steel have low magnetic properties and quickly lose them under the influence of heat, shock and shock.
Alloy steels have better magnetic properties and are used for the manufacture of permanent magnets more often than carbon steel. These steels include chromium, tungsten, cobalt and cobalt-molybdenum.
For the manufacture of permanent magnets, alloys based on iron - nickel - aluminum have been developed in technology. These alloys are characterized by high hardness and brittleness, so they can only be processed by grinding. The alloys have exceptionally high magnetic properties and high magnetic energy per unit volume.
Table 1 shows data on the composition of some hard magnetic materials for the manufacture of permanent magnets.
Chemical composition of magnetically hard materials
| Name of material | Chemical composition in weight percent | Relative weight per unit magnetic energy |
| Carbon steel Chromium steel Tungsten steel Cobalt steel Cobalt-molybdenum steel Alni Alnisi Alnico Magnico | 0.45 C rest Fe 2 – 3 Cr; 1 C 5 W; 1 C 5 – 30 Co; 5 – 8 Cr; 1.5 – 5 W 13 – 17 Mo; 10 – 12 Co 12.5 Al; 25 Ni; 5 CH 14 Al; 34 Ni; 1 Si 10 Al; 17 Ni; 12Co; 6 CH 24 Co; 13 Si; 8 Al; 3 Dc | 26,7 17,2 15,8 5,1 – 12,6 3,8 3,6 3,4 3,1 1 |