High-carbon steel, due to a number of undeniable advantages that it has, is successfully used for the production of products used in many industries. Meanwhile, the use of steels in this category is not always advisable, so it is very important to have a good understanding of the properties and quality characteristics of such alloys.
High carbon steel production
Compound
Depending on the amount of carbon, carbon and alloy steel are divided. The presence of carbon gives the material strength and hardness, and also reduces viscosity and ductility. Its content in the alloy is up to 2.14%, and the minimum amount of impurities due to the manufacturing process allows the bulk to consist of iron up to 99.5%.
High strength and hardness are what characterize carbon steel.
Impurities that are constantly included in the structure of carbon steel have a small content. Manganese and silicon do not exceed 1%, and sulfur and phosphorus are within 0.1%. An increase in the amount of impurities is characteristic of another type of steel, which is called alloyed.
The lack of technical ability to completely remove impurities from the finished alloy allows the following elements to be included in carbon steel:
- hydrogen;
- nitrogen;
- oxygen;
- silicon;
- manganese;
- phosphorus;
- sulfur
The presence of these substances is determined by the steel melting method: converter, open-hearth or other. And carbon is added on purpose. If the amount of impurities is difficult to regulate, then adjusting the level of carbon in the composition of the future alloy affects the properties of the finished product. When the material is filled with carbon up to 2.4%, steel is classified as carbon.
Surface hardening
Main article: Surface hardening
Surface hardening processes harden only the outer surface of a steel part, creating a hard, wear-resistant shell ("casing") but maintaining a tough and ductile interior. Carbon steels are not very hardenable, meaning that they cannot harden thick areas. Alloy steels have better hardenability, so they can be through-hardened and do not require hardening. This property of carbon steel can be beneficial because it gives the surface good wear characteristics but leaves the core flexible and shock absorbent.
Characteristic
The characteristics and structure of the metal are changed using heat treatment, through which the required surface hardness or other requirements for the use of the steel structure are achieved. However, not all structural properties can be adjusted using thermal methods. Such structurally insensitive characteristics include rigidity, expressed by the elastic modulus or shear modulus. This is taken into account when designing critical components and mechanisms in various fields of mechanical engineering.
In cases where the calculation of the strength of an assembly requires the use of small-sized parts that can withstand the required load, heat treatment is used. This effect on “raw” steel makes it possible to increase the rigidity of the material by 2-3 times. The metal that is subjected to this process is subject to requirements regarding the amount of carbon and other impurities. This steel is called high quality.
Content
- 1 Type 1.1 Mild or low carbon steel 1.1.1 High strength steel
- 2.1 Low carbon steel
Classification of carbon steels
According to the direction of application of products, carbon steel is divided into tool and structural.
The last of them is used for the construction of various buildings and frame parts. Tools are used to make durable tools for performing any work, including metal cutting. The use of metal products in the household required the classification of steel into different categories with specific properties: heat-resistant, cryogenic and corrosion-resistant.
According to the method of production, carbon steels are divided into:
- electric steel;
- open hearth;
- oxygen converter.
Differences in the structure of the alloy are due to the presence of different impurities characteristic of a particular smelting method.
The relationship of steel to chemically active environments has made it possible to divide products into:
- boiling;
- semi-calm;
- calm.
Carbon content divides steel into 3 categories:
- hypereutectoid, in which the amount of carbon exceeds 0.8%;
- eutectoid, with a content of 0.8%;
- hypoeutectoid – less than 0.8%.
It is the structure that is a characteristic feature in determining the state of the metal. In hypoeutectoid steels, the structure consists of pearlite and ferrite. Eutectoid ones have pure pearlite, while hypereutectoid ones are characterized by pearlite with admixtures of secondary cementite.
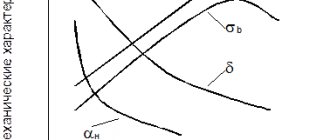
By increasing the amount of carbon, steel increases strength and reduces ductility. The viscosity and brittleness of the material also have a great influence. As the percentage of carbon increases, the impact strength decreases and the fragility of the material increases. It is no coincidence that when the content is more than 2.4%, metal alloys are already classified as cast iron.
According to the amount of carbon in the alloy, steel is:
- low carbon (up to 0.29%);
- medium carbon (from 0.3 to 0.6%);
- high carbon (more than 0.6%).
Steel forging temperature
[20]
| Steel type | Maximum forging temperature | Combustion temperature | ||
| (°F) | (°C) | (°F) | (°C) | |
| 1.5% carbon | 1920 | 1049 | 2080 | 1140 |
| 1.1% carbon | 1980 | 1082 | 2140 | 1171 |
| 0.9% carbon | 2050 | 1121 | 2230 | 1221 |
| 0.5% carbon | 2280 | 1249 | 2460 | 1349 |
| 0.2% carbon | 2410 | 1321 | 2680 | 1471 |
| 3.0% nickel steel | 2280 | 1249 | 2500 | 1371 |
| 3.0% nickel-chromium steel | 2280 | 1249 | 2500 | 1371 |
| 5.0% Nickel (Cemented) Steel | 2320 | 1271 | 2640 | 1449 |
| Chrome vanadium steel | 2280 | 1249 | 2460 | 1349 |
| High speed steel | 2370 | 1299 | 2520 | 1385 |
| Stainless steel | 2340 | 1282 | 2520 | 1385 |
| Austenitic chromium-nickel steel | 2370 | 1299 | 2590 | 1420 |
| Silicon manganese steel spring | 2280 | 1249 | 2460 | 1350 |
Marking
When designating carbon steels of ordinary quality, the letters St are used, which are accompanied by numbers characterizing the carbon content. One digit shows the quantity multiplied by 10, and two digits by 100. When guaranteeing the mechanical composition of the alloy, B is added before the designation, and compliance with the chemical constituents is B.
At the end of the marking, two letters indicate the degree of deoxidation: ps - semi-quiet, kp - boiling state of the alloys. For calm metals this indicator is not indicated. An increased amount of manganese in the structure of the product is designated by the letter G.
When designating high-quality carbon steels used in the manufacture of tools, the letter U is used, next to which a number is written confirming the percentage of carbon in a 10-fold amount, regardless of whether it is two-digit or single-digit. To highlight higher quality alloys, the letter A is added to the designation of tool steels.
Examples of designation of carbon steels: U8, U12A, St4kp, VSt3, St2G, BSt5ps.
References
- ^ a b c d f f d
“Classification of carbon and low-alloy steels” - Knowles, Peter Reginald (1987), Structural Steel Design
(2nd ed.), Taylor and Francis, p. 1, ISBN 978-0-903384-59-9. - Low Carbon Steel Technical Basics Page
- Ehlert, Glenn, Density of Steel
, retrieved April 23, 2009. - Modulus of elasticity, strength properties of metals - iron and steel.
, retrieved April 23, 2009. - Degarmo, p. 377.
- "Low carbon steels." efunda. Retrieved May 25, 2012.
- Ameristeel article on carbon steel Archived October 18, 2006 Wayback Machine
- Nishimura, Naoya; Murase, Katsuhiko; Ito, Toshihiro; Watanabe, Takeru; Novak, Roman (2012). "Ultrasonic detection of spall damage during low-velocity multiple impact." Central European Engineering Journal
.
2
(4): 650–655. Bibcode:2012CEJE…. 2..650N. doi:10.2478/s13531-012-0013-5. - Fundamentals of Engineering page on medium carbon steel
- Fundamentals of Engineering page on high carbon steel
- Smith, page 388
- Alvarenga HD, Van de Putte T, Van Steenberghe N, Sietsma J, Therrien H (October 2014). "The influence of morphology and microstructure of carbides on the kinetics of surface decarburization of C-Mn steels." Metal Mater Trans
A.
46
: 123–133. Bibcode:2015MMTA … 46..123A. Doi:10.1007/s11661-014-2600-у. - Smith, page 386
- Smith, pp. 386–387.
- Smith, pp. 373–377.
- Smith, pp. 389–390.
- Smith, pp. 387–388.
- Smith, page 391
- Brady, George S.; Clauser, Henry R.; Vaccari A, John (1997). Handbook of Materials
(14th ed.). New York, NY: McGraw-Hill. ISBN 0-07-007084-9.
Production
The metallurgical industry produces metal alloys. The specificity of the process for producing carbon steel is the processing of cast iron billets with the reduction of suspended matter such as sulfur and phosphorus, as well as carbon, to the required concentration. The differences in the oxidation technique by which carbon is removed allows us to distinguish different types of smelting.
Oxygen converter method
The basis of the technique was the Bessemer method, which involves blowing air through liquid cast iron. During this process, carbon is oxidized and removed from the alloy, after which the iron ingots gradually turn into steel. The productivity of this technique is high, but sulfur and phosphorus remained in the metal. In addition, carbon steel is saturated with gases, including nitrogen. This improves strength but reduces ductility, making the steel more prone to aging and high in non-metallic elements.
Given the low quality of steel produced by the Bessemer method, it was no longer used. It was replaced by the oxygen-converter method, the difference of which is the use of pure oxygen, instead of air, when purging liquid cast iron. The use of certain technical conditions during purging significantly reduced the amount of nitrogen and other harmful impurities. As a result, carbon steel produced by the oxygen-converter method is close in quality to alloys melted in open-hearth furnaces.
The technical and economic indicators of the converter method confirm the feasibility of such smelting and make it possible to replace outdated methods of steel production.
Open hearth method
A feature of the method for producing carbon steel is the burning of carbon from cast iron alloys not only with the help of air, but also by adding iron ores and rusty metal products. This process usually takes place inside furnaces, to which heated air and combustible gas are supplied.
The size of such melting baths is very large; they can hold up to 500 tons of molten metal. The temperature in such containers is maintained at 1700 ºC, and carbon burning occurs in several stages. First, due to excess oxygen in flammable gases, and when slag forms above the molten metal, through iron oxides. When they interact, slags of phosphates and silicates are formed, which are subsequently removed and the steel acquires the required quality properties.
Steel melting in open hearth furnaces takes about 7 hours. This allows you to adjust the desired composition of the alloy when adding different ores or scrap. Carbon steel has long been manufactured using this method. Such stoves, in our time, can be found in the countries of the former Soviet Union, as well as in India.
Electrothermal method
It is possible to produce high-quality steel with a minimum content of harmful impurities by melting it in vacuum furnaces of electric arc or induction furnaces. Thanks to the improved properties of electric steel, it is possible to produce heat-resistant and tool alloys. The process of converting raw materials into carbon steel occurs in a vacuum, due to which the quality of the resulting workpieces will be higher than the previously discussed methods.
The cost of such metal processing is more expensive, so this method is used when there is a technological need for a high-quality product. To reduce the cost of the technological process, a special ladle is used, which is heated inside a vacuum container.
Alloying additives for steel
Chemical elements belonging to different groups of the periodic table are added to alloy steels.
Alloying metals in the Russian marking of alloy steels are designated using the Cyrillic alphabet. With their help, the qualities of the material are changed:
- Nickel (N)
. An increase in heat capacity, viscosity, and ductility, with a parallel decrease in fragility, which simplifies the processing of metal by pressure. - Chrome (X)
. Increased hardness and impact resistance. The additive provides good protection against rust - it is for this reason that there is always a lot of chromium in stainless steel. - Niobium (B)
. Increased resistance to acids. - Cobalt (K)
. Improvement of indicators such as resistance to shock and high temperatures. - Copper (D)
. Increasing the strength of alloy steel, however, when using this alloying element, the level of viscosity is slightly reduced. This component is usually added to make construction steel. - Titanium (T) and zirconium (Z)
. Reducing the level of grain size, since these metals provide a homogeneous structure, reduces the likelihood of cracking. - Tungsten (B) and molybdenum (M)
. Increased strength during heat treatment, corrosion resistance. - Aluminum (U)
. Increased resistance to scale when exposed to high temperatures. - Vanadium (F)
. Improving the structure, providing higher heat resistance.
Non-metallic additives are also added to alloy steels
:
- Manganese (G)
. Reducing the harmful effects of sulfur, phosphorus and oxygen. - Silicon (C)
. Increased strength while maintaining viscosity. - Selenium (E)
. Increased fluidity, easier mechanical processing. - Boron (R)
. Improved microstructure, increased hardenability. - Nitrogen (A)
. Providing improved mechanical properties - this component is added to high-alloy steels.