Carbide method
You can get acetylene from methane or take calcium carbide as the starting material. The process takes place under normal conditions. When calcium carbide reacts with water, not only acetylene is formed, but also calcium hydroxide (slaked lime). Signs of a chemical process will be the release of gas (hissing), as well as a change in the color of the solution when phenolphthalein is added to a crimson color.
When using technical carbide, which has various impurities, as a starting material, an unpleasant odor is observed during the interaction. It is explained by the presence in the reaction products of such toxic gaseous substances as phosphine and hydrogen sulfide.
Application of acetylene
Acetylene is used in all processes of gas-flame processing of metals (gas welding and gas cutting), due to the high flame temperature, which cannot be achieved when using other combustibles.
For soldering, cutting, surfacing, flame hardening, metallization, gas press welding, welding of non-ferrous metals and alloys, acetylene substitute gases are successfully used:
- propane-butane mixtures
- city gas
- natural gases
- hydrogen
- gasoline vapor
- kerosene vapor
- MAF
- and etc.
In terms of chemical composition, all of them, with the exception of hydrogen, are either compounds or mixtures of various hydrocarbons.
The correct selection and use of substitute gases allows you to achieve high quality welding and cutting, and when gas cutting metals of small thickness, it gives a higher cutting cleanliness.
Gas welding is possible provided that the flame temperature is twice the melting point of the metal being welded. Therefore, substitute gases whose flame temperature is lower than that of acetylene are used for welding metals with a melting point lower than that of steels
For gas cutting, the choice of flammable gas is based on its calorific value, but it must be taken into account that the gas, when burned in a mixture with oxygen, must form a flame with a temperature of at least 2000°C.
The influence of impurities in acetylene on the quality of the weld
Let's look at some more features of the use of acetylene in gas welding - the influence of impurities on the quality of the weld. The following impurities have a harmful effect:
- hydrogen sulfide
- hydrogen phosphide
The above impurities are necessarily removed from acetylene, not only because of the effect on the quality of the weld, but also because of the detrimental effect on the respiratory system and vision of the welder (see the article Explosion hazard, toxicity and self-ignition of acetylene).
Hydrogen sulfide , when burned, forms sulfuric acid, which, when transferred to the weld metal, causes red brittleness. It has been established that the presence of hydrogen sulfide up to 0.007% does not have a harmful effect on the strength of the weld.
Determining the presence of hydrogen sulfide in acetylene is quite easy; you need to hold filter paper soaked in a solution of mercury chloride under a stream of acetylene. In the presence of hydrogen sulfide, the paper will turn white.
The process of purifying hydrogen sulfide is also quite simple - you need to pass acetylene through water, as a result of which hydrogen sulfide will dissolve in water.
Phosphorous hydrogen, when burned, forms phosphoric acid, which, when transferred to the weld metal, causes cold brittleness. It has been established that the presence of hydrogen phosphide up to 0.027% does not have a harmful effect on the strength of the weld.
To determine the presence of hydrogen phosphide, you need to place a piece of filter paper soaked in a ten percent solution of silver nitrate under a stream of acetylene. With a content of hydrogen phosphide of 0.01%, the paper takes on a distinct light yellow color; with a content of more than 0.02%, the paper darkens.
Chemical purification of acetylene from hydrogen phosphide is carried out by passing it through a special cleaning mass - geratol. Geratol is a yellow mass, which, as a result of interaction with hydrogen phosphide, becomes green.
Application of acetylene in the chemical industry
In addition to gas-flame processing, acetylene is used in the chemical industry as the main starting material for the production of a number of important products of organic synthesis: synthetic rubber, plastics, solvents, acetic acid, etc. Next, we will look at how acetylene is used to obtain certain chemical compounds .
Acetaldehyde
The product of the addition of water to acetylene is acetaldehyde. This synthesis was first carried out by M. G. Kucherov in 1881. The reaction proceeds according to the equation:
HC = CH + H2O? CH3 - CHO
The reaction is carried out by passing acetylene through a sulfate solution of mercuric oxide salt at a temperature of 70-80°C.
The use of this reaction was the beginning of the industrial synthesis of organic substances using acetylene as the starting product.
Acetone
When passing a mixture of acetylene and water vapor in a ratio of approximately 1:10 at a temperature of 430-450°C over a zinc-vanadium catalyst, acetone is formed according to the equation:
2C2H2 + 3H2O ? CH3-CO-CH3 + CO2 + H2O
This process has found application on an industrial scale.
Vinyl chloride
When acetylene reacts with hydrogen chloride at 200°C over a catalyst consisting of mercury dichloride supported on activated carbon, vinyl chloride is formed according to the equation:
C2H2 + HCl ? CH2 = CHCl
Vinyl acetate
With acetic acid, also in the presence of mercury salts, acetylene forms vinyl acetate:
C2H3 + CH3COOH ? CH2 = CH-OCO-CH3
Vinyl chloride and vinyl acetate are widely used in the production of plastics.
Cracking of petroleum products
Automotive oscilloscope: concept and principles of operation
Currently, it is not only possible to obtain acetylene from methane. The main industrial method for the production of this representative of alkynes is the cracking (splitting) of hydrocarbons. If acetylene is obtained from methane, then energy costs will be minimal. In addition to inexpensive and accessible raw materials, this technology attracts producers of hydrocarbon raw materials due to the simplicity of the technological equipment used in the process of methane dehydrogenation.
There are two options for carrying out such a chemical process. The first method is based on passing methane through electrodes heated to 1600 degrees Celsius. The technology involves sharp cooling of the resulting product. The second option for dehydrogenating methane to produce acetylene involves the use of energy that is generated during the partial combustion of this alkyne.
Cylinders containing acetylene cannot be equipped with bronze valves, since bronze contains copper. The interaction of this metal with acetylene is accompanied by the production of an explosive salt.
How acetylene was synthesized
Acetylene was first produced in 1836 by Edmund Davy, cousin of the famous Humphry Davy.
He reacted water on potassium carbide: K2C2 + H2O=C2H2 + 2KOH and obtained a new gas, which he called hydrogen bicarbonate. This gas was mainly of interest to chemists from the point of view of the theory of the structure of organic compounds. One of the creators of the so-called theory of radicals, Justus Liebig, called a group of atoms (i.e. radical) C2H3 acetyl. In Latin, acetum means vinegar; the acetic acid molecule (C2H3O+O+H, as its formula was written then) was considered as an acetyl derivative. When the French chemist Marcelin Berthelot in 1855 managed to obtain “hydrogen bicarbonate” by several methods at once, he named it acetylene. Berthelot considered acetylene to be a derivative of acetyl, from which one hydrogen atom was removed: C2H3 – H = C2H2. First, Berthelot obtained acetylene by passing vapors of ethylene, methyl and ethyl alcohol through a red-hot tube. In 1862 he succeeded in synthesizing acetylene from the elements by passing hydrogen through a voltaic arc flame between two carbon electrodes. All the synthesis methods mentioned were only theoretical, and acetylene was a rare and expensive gas until a cheap method was developed for producing calcium carbide by calcining a mixture of coal and quicklime: CaO + 3C = CaC2 + CO. This happened at the end of the 19th century.
Then acetylene began to be used for lighting. In a flame at high temperatures, this gas, containing 92.3% carbon (this is a kind of chemical record), decomposes to form solid carbon particles, which can contain from several to millions of carbon atoms. Heating strongly in the inner cone of the flame, these particles cause the flame to glow brightly - from yellow to white, depending on the temperature (the hotter the flame, the closer its color is to white).
Acetylene torches produced 15 times more light than conventional gas lamps that illuminated the streets. Gradually they were replaced by electric lighting, but for a long time they were used in small lamps on bicycles, motorcycles, and in horse-drawn carriages.
For a long time, acetylene for technical needs (for example, at construction sites) was obtained by “quenching” carbide with water. Acetylene obtained from technical calcium carbide has an unpleasant odor due to impurities of ammonia, hydrogen sulfide, phosphine PH3, arsine AsH3.
Method for producing acetylene from methane
Methane is one of the most accessible and cheapest types of raw materials for organic synthesis. The substances obtained from it are affordable. The production of acetylene from methane (electrocracking) occurs at a temperature of about 1600 degrees. A stream of methane is passed between the two electrodes, which decomposes into acetylene and hydrogen. The main task is to quickly cool the explosive gas and fill white cylinders with it. In them, acetylene is supplied to construction sites and workshops of industrial enterprises. Here it is used together with oxygen: mixing these gases produces a high-temperature flame used for welding and cutting metals.
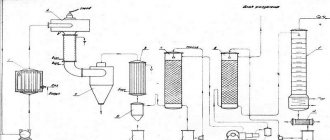
Production of acetylene by pyrolysis method
Pyrolysis acetylene is produced by burning methane mixed with oxygen in reactors at a temperature of 1300-1500°C. The result is a mixture that contains:
- acetylene - up to 8%;
- hydrogen - 54%;
- carbon monoxide - 25%;
- impurities – up to 13%.
Using a solvent (dimethylformamide), acetylene with a concentration of 99.0-99.2% is extracted from it. The remainder of the pyrolysis gases is used to produce ammonia and other products.
Acetylene is also produced by decomposing liquid fuels (oil, kerosene) using an electric arc discharge, which is called electropyrolysis.
Pyrolysis and electropyrolysis of acetylene in its properties is identical to acetylene obtained from calcium carbide, but is 30-40% cheaper.
Analysis of the radical reaction of plasma pyrolysis of methane"
Author — tokarna***@rambler.ru
CONTENT
INTRODUCTION………………………………………………………………………………3
1 Methods for producing acetylene from methane……………………………4
1.1 Physico-chemical properties of substances - participants in the reaction………5
2 Mechanism of hydrogen plasma formation……………………………7
3 Mechanism of the reaction of methane pyrolysis in a jet of hydrogen plasma…….7
4 Study of the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time………………………………………………………………………………………..9
CONCLUSIONS………………………………………………………………………………….12
LIST OF SOURCES USED…………………..13
INTRODUCTION
Modern chemical technology is distinguished by the desire to use increasingly higher operating parameters (temperature, pressure, flow rates of reacting substances), which make it possible to change the passage of chemical reactions in the desired direction. The use of low-temperature plasma opens up broad prospects in this regard.
Pyrolysis of carbon-containing compounds in the plasma of reducing and inert gases is attractive from the point of view of the possibility of obtaining unsaturated compounds and, first of all, acetylene, as well as the possibility of plasma production of high-quality soot.
Almost replaced by ethylene and propylene in the 60s of the last century, acetylene as a chemical raw material has not lost its importance in organic synthesis due to changes in the structure of the fuel balance. Currently, attention to acetylene has again increased. Acetylene is a starting product in the production of vinyl acetate, vinyl chloride, acrylonitrile and other monomers with their subsequent conversion into polymers. The most noticeable increase in acetylene consumption for the synthesis of 1,4-butanediol. In addition, acetylene is quite widely used in welding work (the so-called “balloon” acetylene).
The purpose of this work is to analyze the reaction of methane pyrolysis in a low-temperature plasma flow.
The main objectives of the analysis are to study the properties of all participants in the reaction, analyze the mechanism of the radical reaction and study the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time.
1 Methods for producing acetylene from methane
The most common method for producing acetylene is the oxidative pyrolysis of natural gas or naphtha (low-octane gasoline) at temperatures of 1600-1800 K due to exothermic reactions of hydrocarbon oxidation with oxygen. This process significantly replaces the cumbersome, environmentally harmful and energy-intensive carbide method. However, a comparison of oxidative pyrolysis with plasma-chemical pyrolysis in terms of main indicators demonstrates the advantages of the latter (Table 1).
Table 1 - Comparison of oxidative pyrolysis with plasma-chemical pyrolysis according to the main indicators
| Indicators | Oxidative pyrolysis | Plasma-chemical pyrolysis |
| Consumption of CH4 (t) per 1 t of C2H2 | 7,5 — 8 | 1,7 |
| Selectivity of CH4 to C2H2, % | 30 | 65 — 85 |
| Concentration of C2H2 in pyrolysis gas, % vol. | 10 | 12 — 25 |
| Energy intensity, t.e.* 1 t C2H2 | 11,8 | 6,3 |
*here. – tons of standard fuel
As can be seen from Table 1, the concentration of acetylene in the products of oxidative pyrolysis is two times less than in the plasma-chemical method, the consumption of raw materials per 1 ton of acetylene is almost 2.5 - 3 times higher in the oxidative method compared to the plasma-chemical method, energy intensity (consumption of all types of resources , expressed in t.u.t.) of the plasma-chemical method is almost two times (80%) lower than the oxidative method, the cost of acetylene obtained by the plasma-chemical method is 40% lower compared to the oxidative method. In addition, the plasma-chemical method for producing acetylene does not require the construction of an oxygen plant, which is necessary for the oxidative method.
When choosing one or another method for producing acetylene, it is necessary to assess the raw material base, related industries, and the specifics of the region.
The raw materials in oxidative production are: 1) hydrocarbons; 2) oxygen; 3) water vapor. The by-products of this process—synthesis gas and nitrogen—require their consumers.
The plasma-chemical method requires: 1) a raw material base - carbon-containing substances of various origins and electricity; 2) in the consumer of by-products - hydrogen and soot.
Currently, ethylene production also faces a number of problems with traditional methods of producing it in tube furnaces. The raw materials in these processes are light fractions of petroleum products. However, due to changes in the structure of consumption of petroleum products and increased demand for motor fuel, the main raw materials for the production of ethylene are fuel oil and other heavy fractions, the use of which in traditional technologies is very difficult due to the significant yield of tars and carbon. This also stimulates the introduction of plasma-chemical pyrolysis methods.
The undemanding nature of raw materials—one of the advantages of plasma-chemical processes—gives an additional advantage to this method. A wide range of carbon-containing materials, from methane to coal, were used as raw materials in plasma-chemical pyrolysis to produce acetylene.
1.1 Physicochemical properties of substances participating in the reaction
The main physicochemical properties of the substances participating in the reaction are given in Table 2.
Table 2 - Physico-chemical properties of substances participating in the reaction [3]
| Substance | Melting point, ℃ | Boiling point, ℃ | Application | Toxic effect |
| 1 | 2 | 3 | 4 | 5 |
| CH4 (methane) | -182,5 | -161,58 | As a fuel, for the production of water gas, hydrogen, acetylene, in the production of soot, methyl chloride, hydrocyanic acid. | The first signs of asphyxia (increased heart rate, increased breathing volume, etc.) begin to appear when the oxygen content in the air drops by 25-30%; a mixture of 80% methane and 20% oxygen causes headaches. Hazard class – 4. |
| NS CH (acetylene) | -80,0 | 83,8 | For autogenous welding, for lighting purposes; as a starting product for the production of acetaldehyde, vinyl ethers, vinyl chloride, tetrachloroethane, acrylic acid nitrile, etc. | Strong excitement, followed by a coma, cyanosis, immobility of the pupils, weak and irregular pulse. Hazard class – 4. |
| H2C=CH2 (ethylene) | -169,2 | -103,7 | Raw materials in the production of polyethylene, ethylene oxide, ethyl alcohol, ethanolamines, PVC, thiokol, etc. | The odor is felt at a concentration of 0.02 – 0.026 mg/l. Rapid anesthesia without a noticeable stage of excitation occurs at 80% ethylene with O2. Hazard class – 4. |
| H3C-CH3 (ethane) | -182,8 | -89 | The main use of ethane in industry is the production of ethylene. | Low toxic. Has a narcotic effect. Hazard class – 4. |
| C (soot) | — | 360 – 380 (temperature ignition) | In the production of rubber and printing industry as a dye. | Hazard class – 4. |
| H2 (hydrogen) | -259,14 | -252,8 | For the synthesis of ammonia, urea, methanol; during the hydrogenation of fats, petroleum products, coals and resins; as a reducing agent; for autogenous cutting and welding; for filling balloons, meteorological balloons. | Physiologically inert gas. Causes asphyxiation in very high concentrations due to a decrease in normal oxygen pressure. The narcotic effect occurs at very high pressures. Hazard class – 2. |
All substances involved in the reaction are not highly toxic substances and are widely used in both the chemical and petrochemical industries.
2 Mechanism of formation of hydrogen plasma
A gas, hydrogen, enters the plasmatron between two electrodes. An electric current passes through the electrodes (one is graphite, the other is ferrite). Under the influence of electric current, a molecular hydrogen ion and an electron are formed according to the following scheme:
The resulting electron is transferred to the metal surface.
A molecular hydrogen ion under the influence of an electric current dissociates into a hydrogen ion and a hydrogen atom:
Then the ionization of the hydrogen atom occurs:
This creates a partially ionized gas containing neutral atoms (molecules) and charged particles (ions and electrons).
3 Mechanism of the reaction of methane pyrolysis in a jet of hydrogen plasma
The main type of hydrocarbon raw material for the production of acetylene is natural gas - methane. The reaction takes place at 1500 - 1600 and at atmospheric pressure in a jet of hydrogen plasma, which is a high-temperature coolant.
The main difficulty in producing acetylene by pyrolysis of natural gas is the need to create high temperatures and supply large amounts of heat to the endothermic reaction of acetylene formation from methane.
In general, the reaction for producing acetylene from methane and its molar balance in the plasma flow has the form:
CH4 HC CH + H2C=CH2 + H3C-CH3 + C + CH4 + H2 – 90
1 0,60 0,21 0,05 0,01 0,06 0,77
The temperature of the plasma jet at the beginning of the process drops very quickly due to the transfer of heat to the introduced methane and the occurrence of endothermic reactions of its decomposition.
The formation of acetylene from methane occurs according to the mechanism proposed by Cassel. Active hydrogen atoms that are part of the plasma abstract hydrogen from the methane molecule, forming the corresponding radicals:
It is easier to remove hydrogen from an ethylene molecule, since the C-C bond energy in an ethylene molecule is 101.16, and the C-H bond energy is 85.56.
Open circuit stage:
The thermodynamic stability of acetylene at temperatures above 1100 K is greater than that of other hydrocarbons. However, at very high temperatures there are competitive reactions of compaction and decomposition of acetylene [6, p.64].
NS CH C + H2 + 54.9
In this regard, pyrolysis to produce acetylene is carried out at very short contact times with rapid “hardening” of the reaction products.
4 Study of the dependence of changes in the concentrations of methane decomposition products, temperature and speed of the plasma jet on time
An important prerequisite for the scientific development of the plasma-chemical process of methane pyrolysis is the calculation of the kinetics of this process. A system of equations of hydrodynamics and chemical kinetics of a high-temperature gas jet in which methane decomposes and acetylene is “quenched” was numerically integrated on an electronic computer. The equations of chemical kinetics were written based on the assumption of the formation of acetylene from methane according to the well-known Kassel scheme. The results of solving this system of equations are shown in Figures 1, 2, 3. [1, p.283].
Figure 1 – Dependence of changes in the concentrations of methane decomposition products, temperature and plasma jet speed on time
As can be seen from Figure 1, in the first stages of the reaction there is a rapid decrease in temperature. This decrease in temperature is explained by the occurrence of a mainly endothermic reaction of methane decomposition, which occurs at a very high rate at the initial reaction temperature.
Figure 2 – Dependence of changes in the concentrations of methane decomposition products, temperature and velocity of the plasma jet on time
As can be seen from Figure 2, as the temperature decreases, the rate of methane decomposition drops sharply, and spontaneous inhibition of the reaction occurs, which can be called auto-hardening, in contrast to forced hardening, carried out by special external influences.
Figure 3 – Dependence of changes in the concentrations of methane decomposition products, temperature and velocity of the plasma jet on time
As can be seen from Figure 3, at further stages of the reaction, acetylene decomposes to soot and hydrogen, accompanied by the release of heat and an increase in temperature. To eliminate unwanted decomposition of acetylene, it is necessary to carry out forced hardening. The place, time and rate of forced hardening are determined by the kinetics of the processes under consideration. Kinetic calculations made it possible to estimate the rate of forced temperature decrease required to preserve the formed acetylene. It is ~ 106 degrees/sec.
Since the pyrolysis of methane in a plasma jet of hydrogen may be of industrial interest, hydrogen was also used as a plasma-forming gas in the laboratory setup. The choice of hydrogen as a plasma-forming substance is primarily due to the fact that hydrogen is one of the main reaction products, the volumetric yield of which is three times higher than the volumetric yield of acetylene. Consequently, under industrial production conditions, the process of methane pyrolysis in a hydrogen plasma jet is abundantly supplied with hydrogen as a working fluid for the plasma torch. In addition, at a temperature of about 4500-5000 K, hydrogen, almost completely dissociated into atoms, is an effective coolant and reagent. It also prevents the formation of soot and, in addition, has a significant effect on the chemistry of the process.
CONCLUSIONS
- The reaction of plasma pyrolysis of methane, the target product of which is acetylene, has been studied. However, at temperatures above 1100 K, competitive reactions of acetylene compaction and decomposition occur, and therefore pyrolysis is carried out with rapid “hardening” of the reaction products.
- The dependence of changes in the concentrations of methane decomposition products, the temperature and speed of the plasma jet on time has been studied and it has been established that the temperature of the plasma jet at the beginning of the process drops very quickly due to the transfer of heat to the introduced methane and the occurrence of endothermic reactions of its decomposition. At the end of the process, the temperature rises slightly due to the release of heat as acetylene begins to decompose into carbon and hydrogen.