Oxygen is a necessary element, especially when it comes to gas welding. But it is explosive when it comes into contact with fire, so it is stored in a special cylinder, painted blue, across which the inscription “OXYGEN” is written in black paint. The oxygen cylinder is made of thick sheet metal 6-8 mm thick, like a seamless container in which there are no connecting joints.
By its design, the oxygen cylinder resembles an elongated cylindrical shape, as shown in the photo below, with a convex bottom and an upper spherical neck. A valve is screwed onto the latter, locked with a special ring, on top of which a safety cap is installed. Oxygen is pumped through the valve, and gas is supplied from it for the necessary welding operations. For stable vertical installation, a rectangular metal shoe is put on (pressed into place) on the bottom.
Calculation of oxygen in cylinders
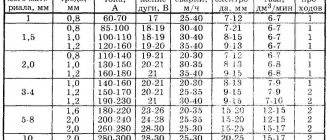
The parameters and dimensions of oxygen cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters.
According to GOST 5583-78 “Gaseous technical and medical oxygen” (Appendix 2), the volume of gaseous oxygen in a cylinder (V) in cubic meters under normal conditions is calculated by the formula:
V = K1•Vb,
Vb — cylinder capacity, dm3;
K1 - coefficient for determining the volume of oxygen in the cylinder under normal conditions, calculated by the formula
K1 = (0.968P + 1) * *
P - gas pressure in the cylinder, measured by a pressure gauge, kgf/cm2;
0.968 — coefficient for converting technical atmospheres (kgf/cm2) into physical ones;
t is the gas temperature in the cylinder, °C;
Z is the oxygen combustion coefficient at temperature t.
The values of the K1 coefficient are given in Table 4, GOST 5583-78.
Let's calculate the volume of oxygen in the most common cylinder in construction: a volume of 40 liters with a working pressure of 14.7 MPa (150 kgf/cm2). Coefficient K1 is determined according to Table 4, GOST 5583-78 at a temperature of 15°C:
V = 0.159 • 40 = 6.36m3
Conclusion (for the case under consideration): 1 oxygen cylinder = 40l = 6.36m3
Table 4. GOST 5583-78.
| Gas temperature in the cylinder, °C | The value of the Ki coefficient at excess pressure, MPa (kgf/cm2) | ||||||||||||||
| 13,7 (140) | 14,2 (145) | 14,7 (150) | 15,2 (155) | 15,7 (160) | 16,2 (165) | 16,7 (170) | 17,2 (175) | 17,7 (180) | 18,1 (185) | 18,6 (190) | 19,1 (195) | 19,6 (200) | 20,1 (205) | 20,6 (210) | |
| -50 | 0,232 | 0,242 | 0,251 | 0,260 | 0,269 | 0,278 | 0,286 | 0,296 | 0,303 | 0,311 | 0,319 | 0,327 | 0,335 | 0,342 | 0,349 |
| -40 | 0,212 | 0,221 | 0,229 | 0,236 | 0,245 | 0,253 | 0,260 | 0,269 | 0,275 | 0,284 | 0,290 | 0,298 | 0,305 | 0,312 | 0,319 |
| -35 | 0,203 | 0,211 | 0,219 | 0,226 | 0,234 | 0,242 | 0,249 | 0,257 | 0,264 | 0,272 | 0,278 | 0,286 | 0,293 | 0,299 | 0,306 |
| -30 | 0,195 | 0,202 | 0,211 | 0,217 | 0,225 | 0,232 | 0,239 | 0,248 | 0,253 | 0,261 | 0,267 | 0,274 | 0,281 | 0,288 | 0,294 |
| -25 | 0,188 | 0,195 | 0,202 | 0,209 | 0,217 | 0,223 | 0,230 | 0,238 | 0,243 | 0,251 | 0,257 | 0,264 | 0,270 | 0,277 | 0,283 |
| -20 | 0,182 | 0,188 | 0,195 | 0,202 | 0,209 | 0,215 | 0,222 | 0,229 | 0,235 | 0,242 | 0,248 | 0,255 | 0,261 | 0,267 | 0,273 |
| -15 | 0,176 | 0,182 | 0,189 | 0,196 | 0,202 | 0,208 | 0,215 | 0,221 | 0,227 | 0,234 | 0,240 | 0,246 | 0,252 | 0,258 | 0,263 |
| -10 | 0,171 | 0,177 | 0,183 | 0,189 | 0,195 | 0,202 | 0,208 | 0,214 | 0,220 | 0,226 | 0,232 | 0,238 | 0,244 | 0,250 | 0,255 |
| -5 | 0,165 | 0,172 | 0,178 | 0,184 | 0,190 | 0,195 | 0,202 | 0,207 | 0,213 | 0,219 | 0,225 | 0,231 | 0,236 | 0,242 | 0,247 |
| 0 | 0,161 | 0,167 | 0,172 | 0,179 | 0,184 | 0,190 | 0,196 | 0,201 | 0,207 | 0,213 | 0,219 | 0,224 | 0,229 | 0,235 | 0,240 |
| +5 | 0,157 | 0,162 | 0,168 | 0,174 | 0,179 | 0,185 | 0,190 | 0,196 | 0,201 | 0,207 | 0,212 | 0,217 | 0,223 | 0,228 | 0,233 |
| +10 | 0,153 | 0,158 | 0,163 | 0,169 | 0,174 | 0,180 | 0,185 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,217 | 0,222 | 0,227 |
| +15 | 0,149 | 0,154 | 0,159 | 0,165 | 0,170 | 0,175 | 0,180 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,216 | 0,221 |
| +20 | 0,145 | 0,150 | 0,156 | 0,160 | 0,166 | 0,171 | 0,176 | 0,181 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,215 |
| +25 | 0.142 | 0,147 | 0,152 | 0,157 | 0,162 | 0,167 | 0,172 | 0,177 | 0,182 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,210 |
| +30 | 0,139 | 0,143 | 0,148 | 0,153 | 0,158 | 0,163 | 0,168 | 0,173 | 0,177 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 | 0,206 |
| +35 | 0,136 | 0,140 | 0,145 | 0,150 | 0,154 | 0,159 | 0,164 | 0,169 | 0,173 | 0,178 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 |
| +40 | 0,133 | 0,137 | 0,142 | 0,147 | 0,151 | 0,156 | 0,160 | 0,165 | 0,170 | 0,174 | 0,178 | 0,183 | 0,188 | 0,192 | 0,196 |
| +50 | 0,127 | 0,132 | 0,136 | 0,141 | 0,145 | 0,149 | 0,154 | 0,158 | 0,163 | 0,167 | 0,171 | 0,175 | 0,180 | 0,184 | 0,188 |
Methane cylinder service life
According to the interstate standard GOST ISO 11439-2014, the service life during which the operation of CNG tanks is considered safe is set by the manufacturer, but this period should not exceed 20 years from the date of production.
In fact, almost all manufacturers of automotive vessels for methane fuel set a service life of 15 years for their products. Factories are allowed to indicate the expiration date directly upon shipment of goods.
In addition, throughout the entire service life it is necessary to undergo periodic inspection of the cylinders:
- the first type at least once every 5 years. This condition also applies to containers made of sheet steel, which is their advantage over composite containers.
- types 2,3,4 at least once every 3 years.
Calculation of propane-butane in cylinders
The parameters and dimensions of oxygen cylinders for propane, butane and their mixtures can be viewed according to GOST 15860-84. Currently, four types of these products are used, with volumes of 5, 12, 27 and 50 liters.
Under normal atmospheric conditions and a temperature of 15°C, the density of propane in the liquid state is 510 kg/m3, and butane 580 kg/m3. Propane in the gas state at atmospheric pressure and temperature 15°C is 1.9 kg/m3, and butane is 2.55 kg/m3. Under normal atmospheric conditions and a temperature of 15°C, 0.392 m3 of gas is formed from 1 kg of liquid butane, and 0.526 m3 from 1 kg of propane.
Let's calculate the weight of the propane-butane mixture in the most common cylinder in construction: volume 50 with a maximum gas pressure of 1.6 MPa. The proportion of propane according to GOST 15860-84 must be at least 60% (note 1 to Table 2):
50l = 50dm3 = 0.05m3;
0.05 m3 • (510 • 0.6 + 580 •0.4) = 26.9 kg
But due to the gas pressure limitation of 1.6 MPa on the walls, more than 21 kg cannot be filled into a cylinder of this type.
Let's calculate the volume of the propane-butane mixture in the gaseous state:
21kg • (0.526 • 0.6 + 0.392 •0.4) = 9.93m3
Conclusion (for the case under consideration): 1 cylinder = 50l = 21kg = 9.93m3
These gases are available from us: propane C3H8
Storage conditions
Gas-filled containers must be stored correctly. Cylinders should be placed in a cool, dry place. Gas tanks should be located so that they are located at a sufficient distance from heating elements and are not exposed to direct sunlight.
Filled containers should also be protected from mechanical impact. Sudden depressurization of the cylinder can result in an explosion, which can cause very serious injury.
Calculation of acetylene in cylinders
The parameters and dimensions of acetylene cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters. The body of an acetylene cylinder differs from the body of an oxygen cylinder in its smaller size.
At a pressure of 1.0 MPa and a temperature of 20 °C, a 40-liter cylinder holds 5–5.8 kg of acetylene by mass (4.6–5.3 m3 of gas at a temperature of 20 °C and 760 mm Hg).
The approximate amount of acetylene in the cylinder (determined by weighing) can be determined by the formula:
Va = 0.07 • E • (P – 0.1)
0.07 – coefficient, which takes into account the amount of acetone in the cylinder and the solubility of acetylene.
E – water volume of the cylinder in cubic dm;
P – pressure in the cylinder, MPa (pressure 1.9 MPa (19.0 kgf/cm2) at 20 °C according to GOST 5457-75 “Dissolved and gaseous technical acetylene”);
0.1 – atmospheric pressure in MPa;
Weight of 1 m3 of acetylene at a temperature of 0°C and 760 mmHg. is 1.17 kg.
Weight of 1 cubic meter of acetylene at a temperature of 20°C and 760 mmHg. is 1.09 kg.
Let's calculate the volume of acetylene in a 40 liter cylinder with a working pressure of 1.9 MPa (19 kgf/cm2) at a temperature of 20°C:
Va = 0.07 • 40 • (1.9 – 0.1) = 5.04 m3
Weight of acetylene in a 40 liter cylinder with a working pressure of 1.9 MPa (19 kgf/cm2) at a temperature of 20°C:
5.04 • 1.09 = 5.5kg
Conclusion (for the case under consideration): 1 cylinder = 40l = 5.5kg = 5.04m3
This gas is available from us: acetylene (C2H2)
Types of methane cylinders
Depending on the materials of manufacture, automobile containers for compressed natural gas (CNG) are divided into types:
Type 1 (KPG-1) - all-metal vessels made of alloy or carbon steel, with a wall thickness of 5.5-9.5 mm (thickness depends on the volume of the tank). They have a seamless design and are made primarily from steel pipes using the hot rolling method. After molding the bottom and neck, the cylinder goes through a number of stages:
- heat treatment (normalization) to impart strength properties
- cooling
- machining with drilling a hole for the valve
- taper thread cutting
- cleaning, descaling
- tests (hydraulic, pneumatic, ultrasonic)
- marking
- coloring
Lightweight CNG-1 cylinders are also produced by deep drawing from sheet steel (without welds). Such containers have all the necessary strength parameters, but at the same time their weight is reduced to 20% in relation to pipe vessels.
Cylinders of the first type are considered very reliable, relatively inexpensive, but they are the heaviest.
Type 2 (KPG-2) – metal-plastic cylinders. Made from thinner steel (4.8-6 mm). Strength is achieved through external reinforcement. Strengthening occurs by the method of continuous winding of a polymer material (aramid, glass, carbon fibers) onto the cylindrical part of the liner (body).
Metal composite tanks CNG-2 are quite reliable and have a relatively low weight. Their disadvantage is their relatively high price.
CNG-2 cylinder
Type 3 (KPG-3) – containers with a metal liner made of light aluminum alloys with an external continuous winding of polymer materials. CNG-3 cylinders are among the lightest and most expensive.
Type 4 (CNG-4) - composite methane cylinders have a non-metal (plastic) liner with a polymer shell over the entire surface. A metal insert is used for the neck. CNG-4 containers are ultra-light, but very expensive.
CNG-4 cylinder
Safety requirements
Although argon is not poisonous, high concentrations of the gas pose a health hazard . When inhaling vapors, a person loses consciousness almost instantly. If the victim is not helped, death occurs within a few minutes.
Atmospheric air contains almost 21% oxygen. In industry, a mixture of argon and oxygen is often used. A low content of the last element can lead to tragic results - from oxygen starvation to suffocation.
According to labor protection requirements, the operation of tanks in rooms with damage to the ventilation system is prohibited . This is due to the fact that the molecular weight of this gas is higher than air, so it accumulates in the lower parts of the premises. The workplace must be equipped with gas analyzers. It is necessary to choose models with determination of oxygen content.
Cylinders must be filled with argon by specialists who have completed training courses in the operation of pressure vessels.
Argon in its liquid state can cause harm to others. Frostbite on unprotected areas of the skin and eye damage are not the entire list of potential dangers.
All cylinders must undergo periodic verification at metrological centers that have permission to conduct the appropriate examination. Operation of the container after the end of the inspection period is prohibited .
Coloring Features
Also read on our website a flock about carbon dioxide cylinders
Each type of gas has its own color. This applies not only to the container itself, but also to the strip, as well as the name of the element contained inside the container. For example, an argon tank should be painted gray and the lettering and stripe should be green. And the brown color, white font and black stripe indicate that it contains helium inside. A complete explanation of the scale used is shown in the figure below.
International requirements for painting containers with industrial gas differ from the standards adopted in the CIS countries, including Russia.
Application area
First of all, it is necessary to understand the technical characteristics of the substance. Argon is a chemical element with the following characteristics:
- transparency;
- lack of specific odor;
- inertia;
- not poisonous;
- not flammable
It is synthesized industrially, at special stations, by rectifying atmospheric air at low temperature . This method also separates oxygen and nitrogen. Argon is considered a by-product of production. The technological cycle of ammonia synthesis is also accompanied by a slight release of this substance.
The main area of application is argon arc welding (TIG), where an inert gas protects the weld pool from the effects of oxygen contained in the air. In addition, the argon cylinder, as well as its contents, serves as one of the components of mixtures that are used for plasma cutting or semi-automatic welding.
These works do not limit the scope of gas application. It is used in the following industries:
- Metallurgical. When melting some types of metal, this element is necessary.
- Electric power. They are filled with gas-discharge lamps.
- Food. As a filler for aerosol containers and food packaging.
- Medical. Mainly for antibacterial treatment.
- Metrological. The purity of the element has found application in the production of various gas analyzers.
The cost of producing argon is much higher than that of nitrogen or carbon dioxide. Therefore, it is used only in the most critical work, for which high demands are placed on quality assessment. When joining metal, the gas supply goes through a reducer for argon welding.