Acetylene gas was discovered back in 1836 by scientist Edmund Davy due to the action of water on calcium carbide. From 1855 to 1862, the French chemist Marcelin Berthelot was able to obtain acetylene, or as it was previously called “hydrogen bicarbonate,” in several ways, and he also gave it the name “acetylene.”
The bright, warm spectrum and hot flame of acetylene began to be used in lamps instead of gas lamps, not only at home, but also for street lighting and even as a lamp for bicycles and carriages.
Mole
All substances are made up of atoms and molecules. In chemistry, it is important to accurately measure the mass of substances that react and are produced as a result. By definition, the mole is the SI unit of quantity of a substance. One mole contains exactly 6.02214076×10²³ elementary particles. This value is numerically equal to Avogadro's constant NA when expressed in units of mol⁻¹ and is called Avogadro's number. Amount of substance (symbol n
) of a system is a measure of the number of structural elements. A structural element can be an atom, molecule, ion, electron, or any particle or group of particles.
Avogadro's constant NA = 6.02214076×10²³ mol⁻¹. Avogadro's number is 6.02214076×10²³.
In other words, a mole is an amount of substance equal in mass to the sum of the atomic masses of atoms and molecules of the substance, multiplied by Avogadro’s number. The unit of quantity of a substance, the mole, is one of the seven basic SI units and is symbolized by the mole. Since the name of the unit and its symbol are the same, it should be noted that the symbol is not declined, unlike the name of the unit, which can be declined according to the usual rules of the Russian language. One mole of pure carbon-12 is equal to exactly 12 g.
Application
Acetylene is one of the most significant hydrocarbons that actively enter into chemical bonds. The use of gas is quite wide:
- fuel for gas cutting and metal welding,
- production of solvents by adding chlorine and chlorine derivatives, the elimination of hydrogen chloride produces a high-quality solvent suitable for dry cleaning fabrics,
- production of polyvinyl chloride (wire insulation, leatherette, pipes, etc.),
- production of other polymers necessary for creating plastics, various rubber mixtures, synthetic fibers,
- creation of explosives.
Molar mass
Molar mass is a physical property of a substance, defined as the ratio of the mass of this substance to the amount of substance in moles. In other words, this is the mass of one mole of a substance. The SI unit of molar mass is kilogram/mol (kg/mol). However, chemists are accustomed to using the more convenient unit g/mol.
molar mass = g/mol
Combustion is a high-temperature exothermic redox reaction.
Gas cylinder volume
The volume of a gas cylinder depends on many factors, but the main ones are where it will be used and the type of gas it contains. In accordance with the requirements of GOST 949, standard gas cylinders with a volume of 0.4 to 50 liters with a working pressure of up to 19.6 MPa are manufactured, intended for the storage and transportation of liquefied, compressed and dissolved gases at temperatures from -50 to +60ºС. Well, GOST 15860 contains requirements for gas cylinders with a capacity of 5, 12, 27 and 50 liters for transportation and storage of liquefied hydrocarbon gases (propane, butane and their mixtures) at pressures up to 1.6 MPa.
The main types and volumes of gas cylinders used in production for storing and transporting gases are presented in the table below:
| Cylinder volume, l | Type of gas | Standard for technical requirements |
| 0,4 | nitrogen ammonia argon butane butylene hydrogen air helium oxygen xenon methane propane and other flammable gases sulfur dioxide carbon dioxide phosgene freon chlorine methyl chloroethyl ethylene welding mixtures | GOST 949 |
| 0,7 | ||
| 1,0 | ||
| 1,3 | ||
| 2,0 | ||
| 2,0 | ||
| 3,0 | ||
| 3,0 | ||
| 4,0 | ||
| 5,0 | ||
| 6,0 | ||
| 7,0 | ||
| 8,0 | ||
| 10,0 | ||
| 12,0 | ||
| 20,0 | ||
| 25,0 | ||
| 32,0 | ||
| 40,0 | ||
| 50,0 | ||
| 5 | propane butane a mixture of propane and butane | GOST 15860 |
| 12 | ||
| 27 | ||
| 50 |
For the storage and transportation of argon, helium, carbon dioxide in welding production, the most widely used are cylinders with a capacity of 40 liters, which are produced in accordance with the requirements of GOST 949. And for propane and butane, gas cylinders with a volume of 50 liters, manufactured in accordance with GOST 15860, are most often used.
Molar mass of elements and compounds
Compounds are substances consisting of different atoms that are chemically bonded to each other. For example, the following substances, which can be found in any housewife’s kitchen, are chemical compounds:
- salt (sodium chloride) NaCl
- sugar (sucrose) C₁₂H₂₂O₁₁
- vinegar (acetic acid solution) CH₃COOH
The molar mass of a chemical element in grams per mole is numerically the same as the mass of the element's atoms expressed in atomic mass units (or daltons). The molar mass of compounds is equal to the sum of the molar masses of the elements that make up the compound, taking into account the number of atoms in the compound. For example, the molar mass of water (H₂O) is approximately 1 × 2 + 16 = 18 g/mol.
Specific gravity of acetylene. Weight of acetylene in 1m3
Acetylene gas was discovered back in 1836 by scientist Edmund Davy due to the action of water on calcium carbide. From 1855 to 1862, the French chemist Marcelin Berthelot was able to obtain acetylene, or as it was previously called “hydrogen bicarbonate,” in several ways, and he also gave it the name “acetylene.”
The bright, warm spectrum and hot flame of acetylene began to be used in lamps instead of gas lamps, not only at home, but also for street lighting and even as a lamp for bicycles and carriages.
Properties of acetylene
In Soviet times, acetylene was used on construction sites by mixing calcium carbide with water. The unpleasant odor that accompanied the process was due to impurities of ammonia and hydrogen sulfide in the technical carbide. Pure acetylene itself:
- gas with a faint ethereal odor,
- slight sweetish taste,
- lighter than air
- slightly toxic.
Molecular mass
Molecular mass (formerly known as molecular weight) is the mass of a molecule, calculated as the sum of the masses of each atom that makes up the molecule multiplied by the number of atoms in that molecule. Molecular mass is a dimensionless
a physical quantity numerically equal to molar mass. That is, molecular mass differs from molar mass in dimension. Although molecular mass is dimensionless, it still has a value called the atomic mass unit (amu) or dalton (Da), which is approximately equal to the mass of one proton or neutron. The atomic mass unit is also numerically equal to 1 g/mol.
Acetylene
Introduction
Acetylene (C2H2) is a chemical gaseous compound of carbon with hydrogen, colorless, with a weak ethereal odor and a sweetish taste.
Acetylene is most widely used in gas welding production due to its important properties for welding (high flame temperature, high calorific value). Thus, during the decomposition of 1 kg of acetylene, 8473.6 kJ of heat is released. This is the only gas whose combustion is possible in the absence of oxygen (or an oxidizing agent at all).
The release of heat during the combustion of acetylene is due to the following processes:
- Acetylene decomposition: C2H2 = 2C + H2
- carbon combustion: 2C + O2 = 2CO, 2CO + O2 = 2CO2
- hydrogen combustion: H2 + 1/2O2 = H2O
Acetylene is lighter than air, the mass of 1 m3 of acetylene at a temperature of 20 ° C (273 K) and normal atmospheric pressure is 1.09 kg. At normal pressure and temperature from –82.4 °C (190.6 K) to –84.0 °C (189 K) acetylene turns into a liquid state, and at a temperature of –85 °C (188 K) it solidifies, forming crystals .
Technical acetylene is available in two types: dissolved and gaseous.
Technical dissolved acetylene grade A is intended to power lighting installations, technical dissolved acetylene grade B and technical gaseous acetylene are intended as a combustible gas for flame processing of metals.
Technical acetylene is produced from calcium carbide by decomposing the latter with water. At the same time, harmful impurities that pollute acetylene pass from calcium carbide into acetylene: hydrogen sulfide, ammonia, phosphorus hydrogen, silicon hydrogen. These impurities can deteriorate the properties of the deposited metal and are therefore removed from acetylene by rinsing in water and chemical cleaning. The admixture of hydrogen phosphide is especially undesirable; a content of more than 0.7% in acetylene increases the explosion hazard of the latter.
Properties of acetylene
The main properties of acetylene are given in table 1.
Table 1
- Basic properties of acetylene
| Index | Indicator data |
| Formula | С2H2 |
| Molecular mass | 26,038 |
| Density (at 0 °C and pressure 760 mm Hg), kg/m3 | 1,17 |
| Density (at 20 °C and pressure 760 mm Hg), kg/m3 | 1,09 |
| Critical temperature, °C | 35,9 |
| Critical pressure, kgf/cm2 | 61,6 |
| Flame temperature, °C | 3150-3200 |
| Boiling point (at 760 mm Hg), °C | -81,8 |
| Melting (solidification) temperature (at 760 mm Hg), °C | -85 |
| Highest specific heat of combustion, kJ/m3 | 58660 |
| Lowest specific heat of combustion, kJ/m3 | 55890 |
| Self-ignition temperature, °C | 335 |
| Self-ignition pressure, MPa | 0,14–0,16 |
In terms of physical and chemical indicators, technical acetylene must comply with the standards specified in Table 2.
Table 2
- Physico-chemical indicators of technical acetylene
| Index | For acetylene | |||
| dissolved | gaseous | |||
| grade A | brand B | |||
| highest quality category | highest quality category | first quality category | ||
| Volume fraction of acetylene, % not less | 99,5 | 99,1 | 98,8 | 98,5 |
| Volume fraction of air and other gases sparingly soluble in water, % not more than | 0,5 | 0,8 | 1,0 | 1,4 |
| Volume fraction of hydrogen phosphide, % not more than | 0,005 | 0,02 | 0,05 | 0,08 |
| Volume fraction of hydrogen sulfide, % no more | 0,002 | 0,005 | 0,05 | 0,05 |
| Mass concentration of water vapor at a temperature of 20 °C and a pressure of 101.3 kPa (760 mm Hg), g/m3, no more What corresponds to the saturation temperature, °C, no more | 0,4 -26 | 0,5 -24 | 0,6 -22 | Not standardized |
Acetylene solubility
Acetylene gas can dissolve in many liquids.
Data on the solubility of acetylene in some liquids at atmospheric pressure and a temperature of 15 ° C are given in Table 3. Table 3
- Solubility of acetylene in liquids
| Solvent | Solubility of acetylene in 1 liter of liquid, l |
| Acetone | 23 |
| Petrol | 5,7 |
| Benzene | 4,0 |
| Water | 1,15 |
The solubility of acetylene in liquids increases with decreasing temperature.
Data on the solubility of acetylene in acetone at various temperatures are given in Table 4. Table 4
- Effect of temperature on the solubility of acetylene in acetone
| Temperature, °C | –20 | –15 | –10 | –5 | 0 | +5 | +10 | +15 | +20 | +30 |
| Solubility, l/l | 52 | 47 | 42 | 37 | 33 | 29 | 26 | 23 | 20 | 16 |
Dissolved acetylene is called acetylene, located in a cylinder filled with a porous mass impregnated with a solvent - acetone. Artificial cooling of the cylinders speeds up the process of filling them. In the pores of the porous mass, acetylene is dissolved in acetone. When the valve of the cylinder is opened, acetylene is released from acetone in the form of a gas. Dissolved acetylene is intended for storage and transportation.
Explosiveness of acetylene
When using acetylene, its explosive properties must be taken into account. This is the only gas widely used in industry, the combustion and explosion of which is possible even in the absence of oxygen or other oxidizing agents.
The auto-ignition temperature of acetylene depends on pressure (Table 5).
Table 5
- Dependence of the auto-ignition temperature of acetylene on pressure
| Absolute pressure, kgf/cm3 (MPa) | 2 (0,2) | 3 (0,3) | 4 (0,4) | 22 (2,2) |
| Self-ignition temperature, °C (K) | 630 (903) | 530 (803) | 475 (748) | 350 (623) |
Increasing the pressure significantly reduces the auto-ignition temperature of acetylene. Particles of other substances present in acetylene increase its contact surface and thereby reduce the auto-ignition temperature at atmospheric pressure to the following values, °C (K):
- iron filings – 520 (793);
- brass shavings – 500–520 (773–793);
- calcium carbide – 500 (773);
- aluminum oxide – 490 (763);
- copper shavings – 460 (733);
- activated carbon – 400 (673);
- iron oxide hydrate (rust) – 280–300 (553–573);
- iron oxide – 280 (553);
- copper oxide – 250 (523).
If acetylene is slowly heated to a temperature of 700–800 °C (973–1073 K) at atmospheric pressure, then its polymerization occurs, during which the molecules become denser and form more complex compounds: benzene C6H6, styrene C8H8, naphthalene C10H8, toluene C7H8, etc. Polymerization is always accompanied by the release of heat and, when acetylene is rapidly heated, it can turn into spontaneous ignition or explosive decomposition.
If, when acetylene is compressed in a compressor to a pressure of 29 kgf/m3 (2.9 MPa), the temperature at the end of this process does not exceed 275 ° C (548 K), then ignition does not occur, which allows filling cylinders with acetone for the purpose of long-term storage and transportation . With increasing pressure, the temperature at which the polymerization process begins decreases (Fig. 1).
| Fig.1. Regions of polymerization (I) and explosive decomposition (II) of acetylene |
In the practical use of acetylene, it is permissible to heat it to the following temperature values, °C (K):
- 300 (573) – at a pressure of 1 kgf/cm2 (0.1 MPa);
- 150–180 (423–453) – at 2.5 kgf/cm2 (0.25 MPa);
- 100 (373) – at higher pressures.
One of the important indicators of the explosiveness of flammable gases and vapors is the ignition energy. The smaller this value, the more explosive the substance is. Acetylene ignition energy values (under normal conditions): with air – 19 kJ; in oxygen – 0.3 kJ.
Water vapor serves as a phlegmatizer for acetylene, i.e. its presence significantly reduces the ability of acetylene to self-ignite in the presence of random heat sources and explosive decomposition. According to current standards for acetylene generators, in which acetylene is always saturated with water vapor, the maximum excess pressure is 150 kPa, and the absolute pressure is 250 kPa.
At atmospheric pressure, a mixture of acetylene with air is explosive if it contains 2.2% acetylene or more, a mixture with oxygen - 2.8% acetylene or more (there are no upper limits on the concentration of acetylene for its mixtures with air and oxygen, since at With sufficient ignition energy, pure acetylene can explode).
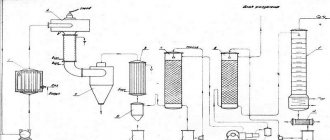
Acetylene production
In industry, acetylene is produced by the decomposition of liquid fuels, such as oil and kerosene, by exposure to an electric arc discharge. A method for producing acetylene from natural gas (methane) is also used. A mixture of methane and oxygen is burned in special reactors at a temperature of 1300–1500 °C. Concentrated acetylene is extracted from the resulting mixture using a solvent. Producing acetylene industrially is 30–40% cheaper than producing acetylene from potassium carbide. Industrial acetylene is pumped into cylinders, where a special mass is dissolved in acetone in the pores. In this form, consumers receive bottled industrial acetylene. The properties of acetylene do not depend on the method of its preparation. The residual pressure in an acetylene cylinder at a temperature of 20 °C should be 0.05–0.1 MPa (0.5–1.0 kgf/cm2). The operating pressure in a filled cylinder should not exceed 1.9 MPa (19 kgf/cm2) at 20 °C.
To preserve the filling mass, acetylene cannot be removed from the cylinder at a speed of 1700 dm3/h.
Let's take a closer look at the method of producing acetylene in a calcium carbide generator. Calcium carbide is produced by fusing coke and quicklime in electric arc furnaces at a temperature of 1900–2300 ° C, at which the reaction occurs:
Ca + 3C = CaC2 + CO
Molten calcium carbide is poured from the furnace into molds where it cools. Next, it is crushed and sorted into pieces ranging in size from 2 to 80 mm. The finished calcium carbide is packaged in hermetically sealed calcium carbide, there should not be more than 3% of particles less than 2 mm in size (dust). According to GOST 1460-81, the dimensions (granulation) of calcium carbide pieces are established: 2 × 8; 8x15; 15×25; 25x80 mm.
When calcium carbide reacts with water, it releases acetylene gas and forms the residue as slaked lime, which is a waste product.
The decomposition reaction of calcium carbide with water occurs according to the following scheme:
| CaC2 | + | 2H2O | = | C2H2 | + | Ca(OH)2 |
| Calcium carbide 1 kg | Water 0.562 kg | Acetylene gas 0.406 kg | Slaked lime 1.156 kg |
From 1 kg of chemically pure calcium carbide, it is theoretically possible to obtain 372 dm3 (liters) of acetylene. Practically, due to the presence of impurities in calcium carbide, the yield of acetylene is up to 280 dm3 (liters). On average, to produce 1000 dm3 (liters) of acetylene, 4.3–4.5 kg of calcium carbide is consumed.
Carbide dust decomposes almost instantly when wetted with water. Carbide dust cannot be used in conventional acetylene generators designed to operate on lump calcium carbide. Specially designed generators are used to decompose carbide dust. For cooling acetylene during the decomposition of calcium carbide. Also used is from 5 to 20 dm3 (liters) of water per 1 kg of calcium carbide. The “dry” method of decomposition of calcium carbide is also used. For 1 kg of finely crushed calcium carbide, 0.2–1 dm3 (liters) of water is supplied to the generator. In this slaking process, lime is obtained not in the form of liquid lime sludge, but in the form of a dry “fluff”, the removal, transportation and disposal of which is greatly simplified.
Transportation and storage
Technical gaseous acetylene is transported through pipelines made of seamless steel pipes in accordance with GOST 8731 and GOST 8734. The acetylene pressure in the pipeline should be no more than 0.15 MPa (1.5 kgf/cm2). Pipeline painting is in accordance with GOST 14202.
The gas pressure in the pipeline must be measured with a pressure gauge of accuracy class 2.5 according to GOST 8625, the dial of which must bear the inscription “Acetylene”.
Technical dissolved acetylene is filled into steel cylinders for dissolved acetylene with a porous mass (active carbon or cast porous mass) and acetylene.
Cylinders must be equipped with special types of valves designed for acetylene cylinders.
The gas pressure in the cylinder must be measured with a pressure gauge of accuracy class no lower than 4 according to GOST 8625. The gas temperature in the cylinder is taken equal to the ambient temperature in which the filled cylinder must be kept for at least 8 hours.
At a nominal pressure of 1.9 MPa (19.0 kgf/cm2) at 20 °C, the gas pressure in the cylinder in the temperature range from minus 5 to plus 40 °C should correspond to that indicated in Table 6. Table 6
-
Acetylene pressure in the cylinder in the range temperatures
| Gas temperature, °C | Gas pressure in the cylinder, MPa (kgf/cm2), no more |
| -5 | 1,34 (13,4) |
| 0 | 1,40 (14,0) |
| +5 | 1,50 (15,0) |
| +10 | 1,65 (16,5) |
| +15 | 1,80 (18,0) |
| +20 | 1,90 (19,0) |
| +25 | 2,15 (21,5) |
| +30 | 2,35 (23,5) |
| +35 | 2,60 (26,0) |
| +40 | 3,00 (30,0) |
The residual gas pressure in the cylinder is measured with a pressure gauge of accuracy class 2.5 with a scale diameter of at least 100 mm according to GOST 8625.
Cylinders from the consumer must be supplied with a residual pressure corresponding to that indicated in Table 7.
Table 7
- Residual pressure of acetylene in the cylinder
| Gas temperature, °C | Residual pressure in the cylinder, MPa (kgf/cm2), not less |
| Up to 0 | 0,05 (0,5) |
| From 0 to +15 | 0,10 (1,0) |
| +15 to +25 | 0,20 (2,0) |
| +25 to +35 | 0,30 (3,0) |
Dissolved acetylene in cylinders is transported by all modes of transport in accordance with the rules for the transportation of dangerous goods in force for this type of transport, and the rules for the design and safe operation of pressure vessels.
By rail, cylinders filled with dissolved acetylene are transported by carload and small shipments in covered wagons. When transporting in small shipments, the cylinder caps must be sealed.
To mechanize loading and unloading operations and consolidate transportation by road, medium-volume cylinders are placed in special metal containers.
When transporting small-volume cylinders by all means of transport, they must be additionally packed in plank lattice boxes of type VII in accordance with GOST 2991. Cylinders must be placed horizontally in the boxes, with valves in one direction, with obligatory gaskets between the cylinders, protecting them from hitting each other.
Cylinders filled with acetylene are stored in special warehouses or in open areas under a canopy that protects them from precipitation and direct sunlight, according to coolant group 2 GOST 15150.
Safety requirements
Acetylene is an explosive gas. Acetylene explosions have great destructive power.
With air it forms an explosive mixture with a lower concentration limit of ignition at atmospheric pressure, reduced to a temperature of 25 ° C, - 2.5% (by volume) according to GOST 12.1.004-85.
Self-ignition temperature 335 °C.
Self-ignition pressure 0.14–0.16 MPa.
According to categories and explosion hazard groups, acetylene belongs to category IIC-T2 according to GOST 12.1.011-78.
Under certain conditions, acetylene reacts with copper, forming explosive compounds, therefore, in the manufacture of acetylene equipment, the use of alloys containing more than 70% copper is strictly prohibited.
The pressure generated during an acetylene explosion depends on the initial parameters and the nature of the explosion. It can increase by approximately 10-12 times compared to the initial value during an explosion in small vessels and increase by 22 times during the detonation of pure acetylene, and 50 times during the detonation of an acetylene-oxygen mixture.
Technical acetylene (with impurities) has a sharp, unpleasant odor; prolonged inhalation of it causes nausea, dizziness and even poisoning. Acetylene has a narcotic effect. Poisoning is caused mainly by hydrogen phosphide contained in acetylene carbide.
Acetylene gas is lighter than air and accumulates in the highest points of poorly ventilated rooms, where the formation of an acetylene-air mixture is possible.
The acetylene content in the air of the working area must be monitored by automatic continuous devices that signal an increase in the permissible explosion-proof concentration of acetylene in the air, as well as periodically using indicator tubes in accordance with GOST 12.1.014-84.
Acetylene production falls into category A for fire hazard, and class B1 for explosive zones; B1a; V1b; V1g.
Acetylene production premises must have supply and exhaust ventilation.
Compressed nitrogen, carbon dioxide fire extinguishers, asbestos sheets, and sand should be used as fire extinguishing agents.