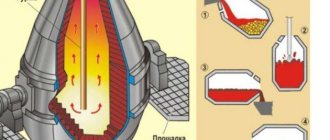
Steel production in converters.
The converter is a pear-shaped vessel. The upper part is called a visor or helmet. It has a neck through which liquid cast iron and steel and slag are drained. The middle part has a cylindrical shape. In the lower part there is an attached bottom, which is replaced with a new one as it wears out. An air box is attached to the bottom, into which compressed air enters.
The capacity of modern convectors is 60 - 100 tons or more, and the air blast pressure is 0.3-1.35 Mn/m. The amount of air required to process 1 ton of cast iron is 350 cubic meters.
Before pouring cast iron, the convector is turned to a horizontal position, at which the tuyere holes are above the level of the poured cast iron. Then it is slowly returned to a vertical position and at the same time a blast is applied, which prevents the metal from penetrating through the holes of the tuyeres into the air box. In the process of blowing air through liquid cast iron, silicon, manganese, carbon and partially iron burn out.
When the required carbon concentration is reached, the convector is returned to a horizontal position and the air supply is stopped. The finished metal is deoxidized and poured into a ladle.
Bessemer process. Liquid cast iron with a fairly high content of silicon (up to 2.25% and higher), manganese (0.6-0.9%), and a minimum amount of sulfur and phosphorus is poured into the converter.
Based on the nature of the reaction occurring, the Bessemer process can be divided into three periods. The first period begins after the blast is started in the converter and lasts 3-6 minutes. Small drops of liquid cast iron fly out of the converter neck along with the gases, forming sparks. During this period, silicon, manganese and partially iron are oxidized according to the reactions:
Si + O2 = SiO2,
2Mn + O2 = 2MnO,
2Fe + O2 = 2FeO.
The resulting ferric oxide partially dissolves in the liquid metal, promoting further oxidation of silicon and manganese. These reactions occur with the release of a large amount of heat, which causes the metal to heat up. The slag turns out to be acidic (40-50% SiO2).
The second period begins after almost complete burnout of silicon and manganese. The liquid metal is heated well enough that favorable conditions are created for the oxidation of carbon by the reaction C + FeO = Fe + CO, which occurs with the absorption of heat. The combustion of carbon lasts 8-10 minutes and is accompanied by a slight decrease in the temperature of the liquid metal. The resulting carbon monoxide burns in air. A bright flame appears above the convector neck.
As the carbon content in the metal decreases, the flame above the neck decreases and the third period begins. It differs from previous periods in the appearance of brown smoke above the neck of the converter. This shows that silicon, manganese and carbon have almost completely burned out of the cast iron and very strong oxidation of iron has begun. The third period lasts no more than 2–3 minutes, after which the convector is turned over to a horizontal position and deoxidizing agents (ferromanganese, ferrosilicon or aluminum) are introduced into the bath to reduce the oxygen content in the metal. Reactions occur in the metal
FeO + Mn = MnO + Fe,
2FeO + Si = SiO2 + Fe,
3FeO + 2Al = Al2O3 + 3Fe.
The finished steel is poured from the convector into a ladle and then sent for casting.
To obtain steel with a predetermined amount of carbon (for example, 0.4 - 0.7% C), blowing the metal is stopped at the moment when the carbon has not yet burned out of it, or you can allow the carbon to completely burn out, and then add a certain amount of cast iron or carbon containing a certain amount of ferroalloys.
BASICS OF CAST IRON AND STEEL TECHNOLOGY
Table 7.1. Physical and mechanical properties of metals and their alloys
| Metal | Tensile strength, MPa | Density, kg/m3 |
| Cast iron | 100…600 | |
| Carbon steel | 200…600 | |
| Alloy steel | 500…1600 | |
| Aluminum alloys | 100…300 | 2500…3000 |
| Titanium alloys | up to 1500 | 4500…5000 |
Metals have high strength, and their bending and tensile strength is almost the same as their compressive strength (in stone materials, bending and tensile strength is 10...15 times lower than compressive strength). Thus, the strength of steel is more than 10 times higher than the compressive strength of concrete and 100...200 times the bending and tensile strength; therefore, despite the fact that the density of steel (7850 kg/m3) is 3 times higher than the density of concrete (2500 kg/m3), metal structures with the same load-bearing capacity are much lighter and more compact than concrete ones. This is also facilitated by the high modulus of elasticity of steel (10 times higher than that of concrete and other stone materials). Light alloy structures are even more effective (table above).
Metals are very technological: firstly, products from them can be produced by various industrial methods (rolling, drawing, stamping, etc.), secondly, metal products and structures are easily connected to each other using bolts, rivets and welding.
However, from a builder's point of view, metals also have disadvantages. The high thermal conductivity of metals requires thermal insulation of metal structures of buildings. Although metals are non-flammable, metal structures of buildings must be specially protected from fire. This is explained by the fact that when heated, the strength of metals sharply decreases and metal structures lose stability and deform. Corrosion of metals causes great damage to the economy (see paragraph 7.10). Metals are widely used in other industries, so their use in construction must be economically justified.
The main method of producing ferrous metals is the extraction of pig iron from ore and its subsequent processing into steel. Scrap metal is also used to produce steel. In recent years, direct production of steel from iron ores has begun to develop.
Iron production. Cast iron is produced in blast furnaces by high-temperature (up to 1900 °C) processing of a mixture of iron ore, solid fuel (coke) and flux. Flux (usually limestone CaCO3) is necessary to molten the waste rock (consisting mainly of SiO2 and Al2O3) contained in the ore and ash from fuel combustion. These components, fused with each other, form blast furnace slag, which is mainly a mixture of silicates and calcium aluminates.
Rice. Blast furnace diagram:
1 - tap hole for releasing liquid cast iron; 2—molten slag; 3
- boot device;
4—
gas outlet pipe;
5—drops of molten cast iron; 6—
drops of molten slag;
7
- lance for air supply;
8—
tap hole for releasing molten slag;
9
- liquid cast iron
A blast furnace is a very large engineering structure. The useful volume of the furnace is 2000...3000 m3, and the daily productivity is 5000...7000 tons. Into the furnace (see figure) from above through the device 3
the charge is loaded, and air is supplied from below through tuyeres 7.
As the charge moves downwards, its temperature rises. Coke, burning under conditions of limited access to oxygen, forms CO, which, interacting with iron oxides, reduces them to pure iron, oxidizing to CO2. Iron melts and at the same time dissolves carbon in itself (up to 5%), turning into cast iron. Molten cast iron 9
flows to the bottom of the furnace, and molten slag
2,
being lighter, is located on top of the cast iron. Cast iron and slag are periodically released through tapholes 1 and 8 into the ladle. For every ton of cast iron, about 0.6 tons of fiery liquid slag is obtained.
Blast furnace slag is a valuable raw material for the production of building materials: Portland slag cement, porous aggregate for concrete - slag pumice, slag wool, slag glass, etc.
Cast iron is mainly (about 80%) used for steel production, the rest of the cast iron is used to produce cast iron products.
Depending on the composition, white and gray cast iron are distinguished. White cast iron is hard and durable, containing a large amount of cementite; in gray, due to the presence of silicon, cementite is not formed and carbon is released in the form of graphite.
Steel production. Steel is produced from cast iron and scrap iron and special additives, including alloying elements, by smelting in open-hearth furnaces, converters or electric furnaces. Steel smelting is a complex process consisting of a number of chemical reactions between the raw material mixture, additives and flue gases. The smelted steel is poured into ingots or processed into billets using the continuous casting method.
Manufacturing of steel products. Steel ingots are a semi-finished product from which the necessary products are obtained using various methods. Pressure treatment of steel is mainly used: the metal is deformed under the influence of applied force, maintaining its acquired shape. There is virtually no waste when processing metal by pressure. To facilitate processing, the steel is often preheated. There are the following types of metal forming: rolling, pressing, drawing, forging, stamping. The most common (the most common processing method is rolling; it processes more than 70% of the resulting steel).
When rolling
a steel ingot is passed between the rotating rolls of a rolling mill, as a result of which the workpiece is compressed, stretched, and depending on.
The profile of the rolling rolls acquires a given shape (profile). Steel is rolled mainly in a hot state. The range of hot-rolled steel is round, square, strip, equal-sided and unequal-sided angle steel, channels, I-beams, sheet piles, pipes, smooth and periodic profile reinforcing steel, etc. During drawing, the workpiece
is sequentially pulled through holes (dies) of a size smaller than the cross-section of the workpiece , as a result of which the workpiece is crimped and stretched. When drawing, a so-called hardening appears in the steel, which increases its hardness. Steel drawing is usually done in a cold state, resulting in products with precise profiles and a clean, smooth surface. The drawing method produces wire, small-diameter pipes, as well as rods of round, square and hexagonal cross-section.
Forging
- processing of hot steel by repeated blows of a hammer to give the workpiece a given shape. Forging is used to produce a variety of steel parts (bolts, anchors, staples, etc.).
Stamping
- a type of forging in which steel, stretched under the blows of a hammer, fills the shape of a die. Stamping can be hot or cold. This method can produce products of very precise dimensions.
Pressing
is the process of squeezing the steel in the container through the outlet hole (hole) of the matrix. The starting material for pressing is casting or rolled billets. This method can produce profiles of various sections, including rods, small diameter pipes and various shaped profiles.
Cold profiling -
the process of deforming sheet or round steel in rolling mills. Bent profiles with different configurations in diameter are produced from sheet steel, and hardened cold-flattened reinforcement is produced from round rods using cold forming machines by flattening.
Properties of steels
The density of steel is 7850 kg/m3, which is approximately 3 times higher than the density of stone materials (for example, ordinary heavy concrete has a density of 2400 ± 50 kg/m3).
The strength and deformation properties of steel are usually determined by tensile testing of the steel. Steel, like other metals, behaves as an elastic-plastic material. Elastic modulus
steel is 2.1 • 105 MPa.
The thermal conductivity of steel, like all metals, is very high and amounts to about 70 W/(m • K), i.e. 50...70 times higher than that of concrete.
The coefficient of linear thermal expansion of steel is 10 • 10-6 K-1, i.e., practically equal to the thermal expansion coefficient of concrete.
The melting point of steel depends on its composition and for ordinary carbon steels is in the range of 1500... 1300°C (cast iron with a carbon content of 4.3% melts at 1150°C).
Temperature resistance A slight loss of strength is observed even when heated above 200 “C; after reaching a temperature of 500...600°C, ordinary steels become soft and sharply lose strength. Therefore, steel structures are not fire-resistant and must be protected from fire, for example, by plastering with cement mortars.
3.8. Non-ferrous metals and alloys.
Aluminum and its alloys. Aluminum is a lightweight silver-white metal with low density (2700 kg/m3). In its pure form, aluminum is soft, ductile, easy to cast and roll, and has a melting point of 657 °C.
Aluminum has
increased resistance to corrosion in air due to the formation of a protective film (A12O3) , and has high thermal and electrical conductivity. Aluminum's tensile strength is 90...120 MPa, relative elongation is 20...30%, hardness HB = 25...30, thermal conductivity coefficient is 200 W (m • °C).
In its pure form, aluminum is used in construction for casting parts, making powders (aluminum paints and gas-forming agents in the production of cellular concrete), foil, and electrical wires. Highly effective insulation (alfol) is made from aluminum foil; it is used as a reflector of heat rays, as well as a decorative material.
By anodic oxidation, architectural parts of various colors are obtained from aluminum alloys.
For construction products, aluminum is used in the form of alloys, which include Cu, Mn, Mg, Si, Fe.
Table 7.1. Physical and mechanical properties of metals and their alloys
| Metal | Tensile strength, MPa | Density, kg/m3 |
| Cast iron | 100…600 | |
| Carbon steel | 200…600 | |
| Alloy steel | 500…1600 | |
| Aluminum alloys | 100…300 | 2500…3000 |
| Titanium alloys | up to 1500 | 4500…5000 |
Metals have high strength, and their bending and tensile strength is almost the same as their compressive strength (in stone materials, bending and tensile strength is 10...15 times lower than compressive strength). Thus, the strength of steel is more than 10 times higher than the compressive strength of concrete and 100...200 times the bending and tensile strength; therefore, despite the fact that the density of steel (7850 kg/m3) is 3 times higher than the density of concrete (2500 kg/m3), metal structures with the same load-bearing capacity are much lighter and more compact than concrete ones. This is also facilitated by the high modulus of elasticity of steel (10 times higher than that of concrete and other stone materials). Light alloy structures are even more effective (table above).
Metals are very technological: firstly, products from them can be produced by various industrial methods (rolling, drawing, stamping, etc.), secondly, metal products and structures are easily connected to each other using bolts, rivets and welding.
However, from a builder's point of view, metals also have disadvantages. The high thermal conductivity of metals requires thermal insulation of metal structures of buildings. Although metals are non-flammable, metal structures of buildings must be specially protected from fire. This is explained by the fact that when heated, the strength of metals sharply decreases and metal structures lose stability and deform. Corrosion of metals causes great damage to the economy (see paragraph 7.10). Metals are widely used in other industries, so their use in construction must be economically justified.
The main method of producing ferrous metals is the extraction of pig iron from ore and its subsequent processing into steel. Scrap metal is also used to produce steel. In recent years, direct production of steel from iron ores has begun to develop.
Iron production. Cast iron is produced in blast furnaces by high-temperature (up to 1900 °C) processing of a mixture of iron ore, solid fuel (coke) and flux. Flux (usually limestone CaCO3) is necessary to molten the waste rock (consisting mainly of SiO2 and Al2O3) contained in the ore and ash from fuel combustion. These components, fused with each other, form blast furnace slag, which is mainly a mixture of silicates and calcium aluminates.
Rice. Blast furnace diagram:
1 - tap hole for releasing liquid cast iron; 2—molten slag; 3
- boot device;
4—
gas outlet pipe;
5—drops of molten cast iron; 6—
drops of molten slag;
7
- lance for air supply;
8—
tap hole for releasing molten slag;
9
- liquid cast iron
A blast furnace is a very large engineering structure. The useful volume of the furnace is 2000...3000 m3, and the daily productivity is 5000...7000 tons. Into the furnace (see figure) from above through the device 3
the charge is loaded, and air is supplied from below through tuyeres 7.
As the charge moves downwards, its temperature rises. Coke, burning under conditions of limited access to oxygen, forms CO, which, interacting with iron oxides, reduces them to pure iron, oxidizing to CO2. Iron melts and at the same time dissolves carbon in itself (up to 5%), turning into cast iron. Molten cast iron 9
flows to the bottom of the furnace, and molten slag
2,
being lighter, is located on top of the cast iron. Cast iron and slag are periodically released through tapholes 1 and 8 into the ladle. For every ton of cast iron, about 0.6 tons of fiery liquid slag is obtained.
Blast furnace slag is a valuable raw material for the production of building materials: Portland slag cement, porous aggregate for concrete - slag pumice, slag wool, slag glass, etc.
Cast iron is mainly (about 80%) used for steel production, the rest of the cast iron is used to produce cast iron products.
Depending on the composition, white and gray cast iron are distinguished. White cast iron is hard and durable, containing a large amount of cementite; in gray, due to the presence of silicon, cementite is not formed and carbon is released in the form of graphite.
Steel production. Steel is produced from cast iron and scrap iron and special additives, including alloying elements, by smelting in open-hearth furnaces, converters or electric furnaces. Steel smelting is a complex process consisting of a number of chemical reactions between the raw material mixture, additives and flue gases. The smelted steel is poured into ingots or processed into billets using the continuous casting method.
Manufacturing of steel products. Steel ingots are a semi-finished product from which the necessary products are obtained using various methods. Pressure treatment of steel is mainly used: the metal is deformed under the influence of applied force, maintaining its acquired shape. There is virtually no waste when processing metal by pressure. To facilitate processing, the steel is often preheated. There are the following types of metal forming: rolling, pressing, drawing, forging, stamping. The most common (the most common processing method is rolling; it processes more than 70% of the resulting steel).
When rolling
a steel ingot is passed between the rotating rolls of a rolling mill, as a result of which the workpiece is compressed, stretched, and depending on.
The profile of the rolling rolls acquires a given shape (profile). Steel is rolled mainly in a hot state. The range of hot-rolled steel is round, square, strip, equal-sided and unequal-sided angle steel, channels, I-beams, sheet piles, pipes, smooth and periodic profile reinforcing steel, etc. During drawing, the workpiece
is sequentially pulled through holes (dies) of a size smaller than the cross-section of the workpiece , as a result of which the workpiece is crimped and stretched. When drawing, a so-called hardening appears in the steel, which increases its hardness. Steel drawing is usually done in a cold state, resulting in products with precise profiles and a clean, smooth surface. The drawing method produces wire, small-diameter pipes, as well as rods of round, square and hexagonal cross-section.
Forging
- processing of hot steel by repeated blows of a hammer to give the workpiece a given shape. Forging is used to produce a variety of steel parts (bolts, anchors, staples, etc.).
Stamping
- a type of forging in which steel, stretched under the blows of a hammer, fills the shape of a die. Stamping can be hot or cold. This method can produce products of very precise dimensions.
Pressing
is the process of squeezing the steel in the container through the outlet hole (hole) of the matrix. The starting material for pressing is casting or rolled billets. This method can produce profiles of various sections, including rods, small diameter pipes and various shaped profiles.
Cold profiling -
the process of deforming sheet or round steel in rolling mills. Bent profiles with different configurations in diameter are produced from sheet steel, and hardened cold-flattened reinforcement is produced from round rods using cold forming machines by flattening.
Properties of steels
The density of steel is 7850 kg/m3, which is approximately 3 times higher than the density of stone materials (for example, ordinary heavy concrete has a density of 2400 ± 50 kg/m3).
The strength and deformation properties of steel are usually determined by tensile testing of the steel. Steel, like other metals, behaves as an elastic-plastic material. Elastic modulus
steel is 2.1 • 105 MPa.
The thermal conductivity of steel, like all metals, is very high and amounts to about 70 W/(m • K), i.e. 50...70 times higher than that of concrete.
The coefficient of linear thermal expansion of steel is 10 • 10-6 K-1, i.e., practically equal to the thermal expansion coefficient of concrete.
The melting point of steel depends on its composition and for ordinary carbon steels is in the range of 1500... 1300°C (cast iron with a carbon content of 4.3% melts at 1150°C).
Temperature resistance A slight loss of strength is observed even when heated above 200 “C; after reaching a temperature of 500...600°C, ordinary steels become soft and sharply lose strength. Therefore, steel structures are not fire-resistant and must be protected from fire, for example, by plastering with cement mortars.
3.8. Non-ferrous metals and alloys.
Aluminum and its alloys. Aluminum is a lightweight silver-white metal with low density (2700 kg/m3). In its pure form, aluminum is soft, ductile, easy to cast and roll, and has a melting point of 657 °C.
Aluminum has
increased resistance to corrosion in air due to the formation of a protective film (A12O3) , and has high thermal and electrical conductivity. Aluminum's tensile strength is 90...120 MPa, relative elongation is 20...30%, hardness HB = 25...30, thermal conductivity coefficient is 200 W (m • °C).
In its pure form, aluminum is used in construction for casting parts, making powders (aluminum paints and gas-forming agents in the production of cellular concrete), foil, and electrical wires. Highly effective insulation (alfol) is made from aluminum foil; it is used as a reflector of heat rays, as well as a decorative material.
By anodic oxidation, architectural parts of various colors are obtained from aluminum alloys.
For construction products, aluminum is used in the form of alloys, which include Cu, Mn, Mg, Si, Fe.
Wax removal
The question of how to obtain cast iron of good quality also comes down to cleaning it from this undesirable element. Sulfur is the main harmful impurity that significantly worsens the properties of the final smelting product. Its main quantity is contained in coke. Sulfur is removed by increasing the lime (CaO) content in the charge and increasing the temperature in the furnace. The reaction in this case looks like this: FeS + CaO = FeO + CaO + Q. Other methods can be used to reduce the percentage of sulfur content in cast iron. For example, sometimes the already smelted material is processed in a discharge chute or a bowl of soda. In this case, the removal of sulfur occurs as a result of the reaction FeS + NaCO3 = FeO + Na2S + CO2.
Characteristics of types of carbon metal
The iron-carbon diagram shows what cast iron is made of. In addition to iron, carbon is present in the form of graphite and cementite.
The composition of the cast iron alloy has varieties:
- White. The carbon present here is in a chemically bound state. The metal is strong, but brittle and therefore difficult to machine. In industry it is used in the form of castings. The properties of the material allow it to be processed with an abrasive wheel. The welding process causes difficulty, since there is a possibility of cracks due to the heterogeneity of the structure. Found application in areas related to dry friction. Has increased heat resistance and wear resistance.
- Half-hearted. It has increased fragility, so it is not widely used.
- Grey. GOST 1412–85 indicates what percentage of impurities this metal contains: 3.5% carbon, 0.8% manganese, 0.3% phosphorus, 0.12% sulfur and up to 2.5% silicon. The carbon present in the platelet form creates low impact strength. The characteristics of the type indicate that the material works better in compression than in tension. When heated sufficiently, it has good weldability.
- Malleable. A ferrite base of this type provides it with high ductility. When broken, it has a black, velvety color. It is obtained from white, which languishes for a long time at a temperature of 800-950 degrees.
- Highly durable. The difference from other types is the presence of spherical graphite. It is obtained from gray after adding magnesium to it.
Read also: Lm358 connection circuit in the charger
Slag formation
Thus, we have found out how cast iron is produced. However, when this material is smelted, another product is obtained that is widely used in the national economy. When melting 1 ton of cast iron, 0.6 tons of slag comes out. The fact is that even refined iron ore contains a fairly large amount of clay. Coke also contains ash. To remove these unnecessary elements, among other things, fluxes (calcium and magnesium carbonates) are added to the charge. During the smelting process, they enter into a chemical reaction with various types of impurities, resulting in the formation of slag. It is an aluminosilicate or silicate melt.
The density of slag is less than that of liquid cast iron. Therefore, during the melting process it is located underneath. It is removed periodically through a separate taphole, called a slag taphole. This by-product of iron foundries is used mainly for the production of cement and building blocks as a filler.
Areas of application
This material is used in various industries:
- Mixtures and homogeneous metal are used in mechanical engineering. Internal parts, housings, and moving mechanisms are made from them.
- Shipbuilding, aircraft manufacturing, rocketry.
- Construction - production of fasteners, consumables.
- Instrument making - manufacturing electronics for the home.
- Radioelectronics - creation of elements for electrical appliances.
- Medicine, machine tool building, chemical industry.
- Making weapons.
If a homogeneous material is not suitable for something, compounds based on it, the characteristics of which differ significantly, will do.
Mining
There are several methods for extracting ore. The one that is found most economically feasible is used.
- Open method of development - or quarry. Designed for shallow mineral rock. For mining, a quarry is dug to a depth of up to 500 m and a width depending on the thickness of the deposit. Iron ore is extracted from the quarry and transported by vehicles designed to carry heavy loads. As a rule, this is how high-grade ore is mined, so there is no need to enrich it.
- Mine - when the rock occurs at a depth of 600–900 m, shafts are drilled. Such development is much more dangerous because it involves underground blasting: the discovered layers are blasted, and then the collected ore is transported upward. Despite its dangers, this method is considered more effective.
- Hydraulic mining - in this case, wells are drilled to a certain depth. Pipes are lowered into the mine and water is supplied under very high pressure. The water jet crushes the rock, and then the iron ore is lifted to the surface. Borehole hydraulic production is not widespread, as it requires high costs.
Next, the technology and iron manufacturing processes are discussed.
History of discovery
Everyone remembers the “Iron Age” from school. This is the period in history when man first learned to extract this metal from ore. The Iron Age spans the period from the 9th to the 7th century BC. This metal had a huge influence on the development of people of that time. According to its characteristics, it has replaced mixtures of non-ferrous metals. Tools, weapons, armor, materials for construction and much more were made from it. Gradually, blacksmiths began to mix it with other metals to create new materials. This is how new alloys appeared.
Individual properties of metal
The material is characterized by certain characteristics. These include:
- Physical. Values such as specific gravity or expansion coefficient depend on the carbon content of the metal. The material is heavy, so cast iron bathtubs can be made from it.
- Thermal. Thermal conductivity allows you to accumulate heat and retain it, distributing it evenly in all directions. This is used in the manufacture of frying pans or radiators for heating.
- Mechanical. These characteristics vary depending on the graphite base. The most durable is gray cast iron with a perlite base. A material with a ferrite component is more malleable.
Depending on the presence of impurities, a difference in the properties of the material appears.
These elements include sulfur, phosphorus, silicon, manganese:
- Sulfur reduces the fluidity of the metal.
- Phosphorus reduces strength, but makes it possible to manufacture products of complex shapes.
- Silicon increases the fluidity of the material, lowering its melting point.
- Manganese gives strength, but reduces fluidity.
Agglomeration process
In fact, we’ll look at how cast iron is produced below. Now let's talk about how ore is prepared for smelting directly at metallurgical plants.
If ordinary crushed material is used for smelting, the productivity of the blast furnace will drop sharply. The fact is that such a charge has a low degree of gas permeability. Therefore, before loading into the blast furnace, ore must undergo an agglomeration process.
This procedure is carried out in specialized workshops of metallurgical plants and is a process of sintering rock into pieces of a certain size most suitable for smelting cast iron. Adhesion occurs at a high temperature sufficient to easily melt the surface of the charge particles. As a result, the latter simply stick together to form pieces. In this case, the ore is first mixed with coal. As a result of the combustion of the latter, the temperature necessary for obtaining pieces is achieved. The agglomeration process is stimulated by passing air flows through the layer of ore with coal (from top to bottom).
Not only ore can be used to obtain sinter. Sometimes it is also made from small pieces of iron. Its alloy with what substance makes it possible to obtain cast iron will be discussed below. Of course, it is not pig iron that is used to produce this metal. Regular scrap metal is melted down into cast iron.
Mining and processing plants
The main raw material used in the production of cast iron is iron ore. It is mined in quarries in different places in our country. As you know, mined ore contains a large amount of various types of impurities. Of course, it cannot be used for melting cast iron in such a “raw” form. Therefore, at the first stage, it goes to special enterprises - mining and processing plants. Here waste rocks are removed from it and crushed. Then the clean ore is loaded into train cars and sent to metallurgical plants.