Thermophysical properties of cast iron
The coefficient of linear expansion α, specific heat c and thermal conductivity λ depend on the composition and structure of cast iron, as well as on temperature.
Therefore, their values are given in the appropriate temperature range. With increasing temperature, the values of α and c usually increase, and λ decreases (Table 1). Table 1. Thermophysical properties of gray cast iron depending on temperature
| Temperature, °C | α, 1/°C | s, J/(kg∗°C) | λ, W/(m∗°C) |
| 60 | 10,0 | 502 | 54,4 |
| 160 | 11,0 | 523 | 50,2 |
| 260 | 13,1 | 553 | 48,1 |
| 360 | 13,7 | 586 | 46,0 |
| 510 | 15,9 | 620 | — |
The linear expansion coefficient α and specific heat c of real heterogeneous structures, including cast iron, can be determined by the mixing rule:
Table 2. Thermophysical properties of the structural components of cast iron
| Structural component | α 100 200, 1/°C | c 100 ,J/(kg∗°C) | λ 100 W/(m∗°C) |
| Ferrite | 12,0-12,6 | 460-470 | 72,8-75,5 |
| Austenite | 18-19 | 502 | 41,8 |
| Cementite | 6,0-6,5 | 615 | 49,0 |
| Perlite | 10,0-11,6 | 486 | 50,3-51,9 |
| Graphite | 1,4-3,7 | 795 | 355,8 |
The thermal conductivity of alloys and mixtures, in contrast to the coefficient α and heat capacity c, cannot be determined using the mixing rule. The influence of individual elements on thermal conductivity can only be determined approximately by calculation.
The coefficient α and specific heat capacity c are mainly influenced by the composition of the cast iron, and the thermal conductivity λ is influenced by the degree of graphitization, structure dispersion, non-metallic inclusions, etc.
The coefficient of linear expansion determines not only changes in dimensions depending on temperature, but also the stresses generated in the castings. Reducing α is useful from this point of view and facilitates the conditions for obtaining high-quality castings. But in the case of joint work of cast iron parts with parts made of non-ferrous alloys or other materials that have a higher coefficient of linear expansion, it is necessary to strive to increase the value of α for cast iron.
Heat capacity and thermal conductivity are of great importance for such castings as heating pipes, molds, parts of refrigeration units and internal combustion engines, etc., since they determine the uniformity of temperature distribution in the castings and the intensity of heat removal.
In table Table 3 shows the thermophysical properties of cast irons of various groups.
Table 3. Thermophysical properties of cast iron
| Cast iron | α20 100 ∗10 6 , 1/°C | c20 100 , J/(kg∗°C) | c20 1000 , J/(kg∗°C) | λ20 100 , W/(m∗°C) |
| Gray with flake graphite (GOST 1412-85): | ||||
| SCH10-SCH18 | 10-11 | 502-544 | 586-628 | 46,0-54,4 |
| SCh20-SCh30 | 10-11 | 502-544 | 586-628 | 41,8-50,2 |
| SCH35 | 11,5-12,0 | 502-544 | 628-670 | 37,6-46,0 |
| High-strength (GOST 7293-85): | ||||
| HF 35-HF 45 | 11,5-12,5 | 460-502 | 586-628 | 37,6-46,0 |
| HF 60-HF 80 | 10-11 | 502-523 | 628-670 | 33,5-41,9 |
| HF 100 | 9-10 | 523-565 | 628-670 | 29,3-37,6 |
| Malleable (GOST 7769-82): | ||||
| CC 30-6/CC 37-12 | 10,5-11,0 | 460-511 | 586-628 | 54,4-62,8 |
| CC 45-5/CC 65-3 | 10,3-10,8 | 527-544 | 628-670 | 50,2-54,4 |
| Alloyed (GOST 7769-82) | ||||
| Nickel ChN20D2Sh | 17-19 | — | 460-502 | 17,4 |
| with 35-37% Ni | 1,5-2,5 | — | — | — |
| chromic: | ||||
| CHH16 | — | — | — | 32,5 *1 |
| CHH22 | — | — | — | 25,5 *1 |
| CHH28 | 9-10 | — | — | 17,4 *1 |
| CHH32 | 9-10 | — | — | 19,8 *1 |
| siliceous: | ||||
| ChS5 | 14-17 *2 | — | — | 21,0 *3 |
| ChS15, ChS17 | 4,7 *1 | — | — | 10,5 |
| aluminum: | ||||
| CHYU22SH | 17,5 *1 | — | — | 15,1-28,0 *3 |
| CHY30 | 22-23 *2 | — | — | — |
| *1In the range of 20-200 °C. | ||||
| *2In the range of 20-900 °C. | ||||
| *3In the range of 20-500 °C. | ||||
Heat capacity of cast iron and steel
Steel is considered an alloy of iron with other chemical compounds. Among the components included in the composition, carbon is present in an amount of 2.14%.
Thanks to its presence, iron alloys acquire their strength. The specific gravity of steel is 75500–77500 N/m³. The alloy may sometimes contain alloying elements.
The specific heat capacity of steel at 20 °C is measured at 460 J/(kg*°C), or 110 cal/(kg*°C).
Classification
There are various parameters according to which the material in question is characterized. For example, steel can be used for instrumental or structural purposes. High-speed alloy is considered one of the types of tooling.
There are also differences according to the chemical composition. Depending on what elements are present in the alloy, they are divided into alloyed and carbon. A classification based on the level of carbon concentration has also been adopted.
So, there are three types of alloys:
1. Low carbon. It contains carbon content up to 0.25%.
2. Medium carbon steel. This alloy contains about 0.25-0.6% carbon.
3. High carbon steel. This alloy contains about 0.6-2% carbon.
Alloy steel is classified in a similar way according to the percentage of alloying elements:
1. Low alloy steel contains up to 4%.
2. In a medium alloy alloy, up to 11% is present.
3. High alloy steel. It contains more than 11%.
Steel is produced using various methods and using special technologies. Depending on one or another method, the alloy contains different metallic inclusions. This indicator affects the specific gravity of steel. When classifying alloys according to the amount of impurities, they distinguish:
1. Mixtures of ordinary quality.
2. High quality.
3. High quality.
4. Particularly high quality.
There is also a classification according to the structural composition of the material. For example, ferritic, bainitic, austenitic, pearlitic and martensitic alloys are produced. Undoubtedly, the structural composition also affects the specific gravity of steel.
Alloys are also divided into two-phase and multiphase. This depends on the presence of phases in the structure. Alloys are also classified according to the nature of solidification and the degree of deoxidation. So, there is calm, semi-calm and boiling steel.
Steel production methods
Cast iron is used as a raw material for making steel. The presence of large amounts of carbon, phosphorus and sulfur in its composition makes it brittle and brittle.
To process one material into another, it is necessary to reduce the content of these substances to the desired concentration. At the same time, both the specific gravity of the steel and its properties will change.
This or that method of producing alloys involves different ways of oxidizing carbon in cast iron. Most often used:
1. Open hearth method of steel smelting. It should be noted that this option has recently competed poorly with other methods.
2. Converter method. Today, most steel products are manufactured using this technology.
3. Electrothermal is one of the advanced technological methods for producing steel. As a result, the produced material is of very high quality.
Converter method
Using this technological method, excess cast iron, phosphorus and sulfur are oxidized with oxygen. It is blown under pressure through the molten material in a special furnace. It's called a converter.
This oven is shaped like a pear. The inner part is lined with refractory bricks. This oven is highly mobile: it can rotate 360 degrees. The converter capacity is about 60 tons.
As a rule, two types of raw materials are used for lining:
1. Dinas – it contains SiO2, which has acidic properties.
2. Dolomite mass – MgO and CaO. It is obtained from dolomite material MgCO3*CaCO3, which has the properties of bases.
Due to the different lining materials, converter furnaces are divided into Thomas and Bessemer. The blown air under pressure covers the entire area of the metal.
It should be noted that the processes occurring in the oven last no more than 20 minutes. The length of time the material remains in the converter affects the heat capacity of the steel.
The alloy produced in converter furnaces often contains large amounts of iron monoxide. This is why the material is often of poor quality.
Open hearth furnace
This method of processing cast iron is outdated. Undoubtedly, when using somewhat backward technologies during processing, the quality of the material significantly decreases, its technical characteristics (heat capacity of steel, etc.) change. An open hearth furnace is a large melting bath.
It is covered with a vault of refractory bricks and recuperator chambers. These compartments are designed to heat flammable gas and air. They are filled with a brick (fireproof) nozzle. A stream of hot gas and air is blown into the furnace through the third and fourth recuperators. Meanwhile, the first and second ones are heated by furnace gases.
After a sufficient increase in temperature, the whole process goes in the opposite direction.
Electrothermal method
This method has a number of advantages over open-hearth and converter methods. The electromechanical method allows you to change the chemical composition of the resulting steel. In this case, the mixture after the processing process is of very high quality.
Due to limited air access in electric furnaces, the amount of iron monoxide decreases. It is known to contaminate steel with its impurities. And this, in turn, has a significant impact on its quality. In an electric furnace, the temperature does not drop below 2000 °C.
Thus, harmful impurities such as sulfur and phosphorus are completely removed from the cast iron.
Furnace operating method
Electrothermal furnaces, due to their high temperature, allow steel to be alloyed with refractory metals. These include, in particular, tungsten and molybdenum. The electric steel-melting method makes it possible to obtain a high-quality mixture: the specific heat capacity of steel, as well as its quality characteristics, are at the highest level.
Linear expansion coefficient α
Linear expansion coefficient α. Carbon has the greatest influence on the α coefficient, especially in the bound state. One percent of carbon corresponds to approximately 5 times more cementite than graphite. Therefore, graphitizing elements (Si, Al, Ti, Ni, Cu, etc.) increase, and anti-graphitizing elements (Cr, V, W, Mo, Mn, etc.) reduce the coefficient of linear expansion,
The highest α values are found in austenitic nickel cast irons, as well as ferritic aluminum cast irons such as chugal and pyroferal. Therefore, at a sufficiently high content of Ni, Cu, Mn, the value of α; increases sharply. However, at a Ni content of >20% α decreases: and reaches a minimum at 35–37% Ni. The shape of graphite significantly affects the coefficient of linear expansion only at low temperatures; The α of ductile iron with nodular graphite is slightly higher than the α of ductile iron with flake graphite.
Heat capacity of cast iron and steel - Metals, equipment, instructions
Cast iron consists of carbon, iron and some impurities. It is one of the main materials of ferrous metallurgy. Cast iron is used in the manufacture of household and utility items, machine parts and in other industries. It is used in production, focusing and taking into account its properties and characteristics.
This article is precisely intended to tell you about the density of high-strength, liquid, white and gray cast iron, its melting points and specific heat capacity will also be considered separately.
Cast iron, like any metal, has the following properties: thermal, physical, mechanical, hydrodynamic, electrical, technological, chemical. Let's look at each properties in more detail.
This video talks about the structure and composition of cast iron alloys and the dependence of their properties on a specific composition:
Thermal conductivity of cast iron.
The thermal conductivity of cast iron, to a greater extent than other physical properties, depends on the structure, its dispersion and the smallest impurities, i.e. it is a structure-sensitive property.
Graphitization increases thermal conductivity; therefore, elements that increase the degree of graphitization and the size of graphite are increased, and elements that prevent graphitization and increase the dispersion of structural components are decreased. The indicated effect of graphitization is less for spherical graphite (see Table 4).
The shape of graphite, its precipitation and distribution also affect thermal conductivity. For example, ductile iron has a lower thermal conductivity than gray cast iron. The thermal conductivity of cast iron with compacted graphite (CGI) is higher than that of cast iron and is close to λ of gray cast iron with flake graphite.
High-alloy cast irons are characterized, as a rule, by lower thermal conductivity than ordinary ones.
Specific heat capacity of steel
The summary table shows the specific heat capacity of steel of common grades: carbon, low- and high-alloy steels, as well as cast iron at different temperatures.
The values of the average specific heat capacity of low-alloy steels and carbon steels at various temperatures are given, and the heat capacity of high-alloy steels with special properties depending on temperature is indicated.
The table shows that the specific heat capacity of steel increases with increasing temperature. It should be noted that the heat capacity of steel at room temperature ranges from 440 to 550 J/(kg deg); The specific heat capacity of steel in the table is presented in the temperature range from 20 to 1000°C.
Specific heat capacity of steel at different temperatures Steel grade Temperature, °Steel heat capacity, J/(kg deg)
| 02Х17Н11М2 | 20…400…600…800 | 470…560…610…650 |
| 02X22N5AM3 | 20…100…200…300…400 | 480…500…530…550…590 |
| 03X24N6AM3 (ZI130) | 20…100…200…300…400 | 480…500…530…550…570 |
| 05ХН46МВБЧ (DI65) | 100…200…300…400…500…600…700…800 | 445…465…480…490…500…510…515…520 |
| 06Х12Н3Д | 100…200…300…400 | 523…544…577…594 |
| 07Х16Н6 (Х16Н6, EP288) | 100…200…300…400…500…600…700 | 440…500…550…590…630…670…710 |
| 08 | 100…200…400…600 | 465…477…510…565 |
| 08kp | 100…200…300…400…500…600…700…800…900 | 482…498…514…533…555…584…626…695…695 |
| 08Х13 (0Х13, EI496) | 20 | 462 |
| 08Х14МФ | 20…100…200…300…400…500…600 | 460…473…502…540…574…682…754 |
| 08Х17Т (0Х17Т, EI645) | 20 | 462 |
| 08Х17Н13М2Т (0Х17Н13М2Т) | 20 | 504 |
| 08Х18Н10 (0Х18Н10) | 20 | 504 |
| 08Х18Н10Т (0Х18Н10Т, EI914) | 20…100…200…300…400…500…600…700 | 461…494…515…536…549…561…574…595 |
| 08GDNFL | 100…200…300…400…500…600…700…800…900 | 483…500…517…529…554…571…613…697…693 |
| 09X14N19V2BR1 (EI726) | 20 | 502 |
| 015Х18М2Б-VI (EP882-VI) | 100…200…300…400 | 473…519…578…636 |
| 1Х14Н14В2М (ЭИ257) | 20…100…200…300…400…500…600…700 | 461…486…515…536…544…557…590…624 |
| 4Х5МФ1С (EP572) | 20…100…200…300…400…500…600…700…800 | 431…477…519…565…620…703…888…766…749 |
| 10 | 100…200…400…600 | 465…477…510…565 |
| 10kp | 100…200…400…600 | 466…479…512…567 |
| 10Kh12N3M2FA(Sh) (10Kh12N3M2FA-A(Sh)) | 100…200…300…400…500 | 510…538…562…588…627 |
| 10Х13Н3М1Л | 20 | 495 |
| 10Х17Н13М2Т (Х17Н13М2Т, EI448) | 20 | 504 |
| 10Х17Н13М3Т (Х17Н13М3Т, EI432) | 20 | 504 |
| 10Х18Н9Л | 100 | 504 |
| 10GN2MFA, 10GN2MFA-VD, 10GN2MFA-Sh | 100…200…300…400 | 469…553…599…628 |
| 12MH | 20…200…300…400…500…600…700 | 498…519…569…595…653…733…888 |
| 12X1MF (EI575) | 100…200…300…400…500…600…700…800 | 507…597…607…643…695…783…934…1025 |
| 12Х13 (1Х13) | 20…100…200…300…400…500…600…700…800 | 473…487…506…527…554…586…636…657…666 |
| 12X13G12AS2N2 (DI50) | 100…200…300…400…500…600…700 | 523…559…602…613…648…668…690 |
| 12Х18Н9 (Х18Н9) | 20 | 504 |
| 12Х18Н9Т (Х18Н9Т) | 20…100…200…300…400…500…600…700…800 | 469…486…498…511…519…528…532…544…548 |
| 12Х18Н12Т (Х18Н12Т) | 20…100…200…300…400…500…600…700 | 461…494…515…540…548…561…574…595 |
| 14Х17Н2 (1Х17Н2, EI268) | 20 | 462 |
| 15 | 100…200…400…500 | 469…481…523…569 |
| 15G | 100…300…500 | 496…538…592 |
| 15K | 100…200…400…500 | 469…482…524…570 |
| 15kp | 100…200…300…400…500…600…700…800 | 465…486…515…532…565…586…620…691 |
| 15L | 100…200…400…600 | 469…477…515…570 |
| 15Kh2NMFA-A, 15Kh2NMFA-A class 1 | 100…200…300…400 | 490…515…540…569 |
| 15X11MFBL (1X11MFBL, X11LA) | 100…200…300…400…500…600 | 494…528…574…641…741…867 |
| 15Х25Т (Х25Т, EI439) | 20 | 462 |
| 15ХМ | 100 | 486 |
| 17Х18Н9 | 20 | 504 |
| 18Kh11MNFB (2Kh11MNFB, EP291) | 100…200…300…400…500…600 | 490…540…590…666…766…900 |
| 18ХГТ | 100…200…300…400…500…600…700…800 | 495…508…525…537…567…588…626…705 |
| 20 | 100…200…400…500 | 469…481…536…569 |
| 20G | 100…200…400…500 | 469…481…536…569 |
| 20GSL | 100…200…400…500 | 469…482…536…569 |
| 20K | 100…200…400…500 | 469…482…524…570 |
| 20L | 100…200…400…600 | 469…481…536…570 |
| 20kp | 100…200…300…400…500…600…700…800…900 | 486…498…514…533…555…584…636…703…695 |
| 20HML | 100…200…300…400…500 | 498…572…588…612…660 |
| 20ХМФЛ | 100…200…300…400…500…600 | 498…574…590…615…666…741 |
| 20Х3МВФ (EI415, EI579) | 100…200…300…400…500…600 | 502…561…611…657…716…754 |
| 20Х23Н13 (Х23Н13, EI319) | 20 | 538 |
| 20Х23Н18 (Х23Н18, EI417) | 20 | 538 |
| 20ХН3А | 100…200…300…400…500…600…700…800 | 494…507…523…536…565…586…624…703 |
| 22K | 100…200…400…500 | 469…481…519…569 |
| 25 | 100…200…400…500 | 469…482…524…570 |
| 25L | 100…200…400…600 | 469…481…519…570 |
| 25Х1МФ | 20 | 461 |
| 25Х2М1Ф (EI723) | 100…200…300…400…500…600 | 536…574…607…632…674…733 |
| 25ХГСА | 20…100…200…300…400…500…600…700 | 496…504…512…533…554…584…622…693 |
| 30 | 100…200…300…400…500 | 469…481…544…523…762 |
| 30G | 100…200…300…400…500 | 469…481…544…599…762 |
| 30L | 100…200…400…600 | 469…481…523…570 |
| 30Х13 (3Х13) | 20…100…200…300…400…500…600…700…800 | 473…486…504…525…532…586…641…679…691 |
| 30ХГТ | 100…200…300…400…500…600…700…800 | 495…508…525…537…567…588…626…705 |
| 30X | 20…100…200…300…400…500…600…700…800…900 | 482…496…513…532…555…583…620…703…687…678 |
| 30ХН2МВА (30ХН2МВА) | 20…100…200…300…400 | 466…508…529…567…588 |
| 30ХН3А | 100…200…300…400…500…600… 700…800…900…1000 | 494…504…518…536…558…587… 657…703…695…687 |
| 33ХС | 20…100…200…300…400…500…600…700 | 466…508…529…563…599…622…634…664 |
| 35 | 100…200…400…500 | 469…482…524…570 |
| 35L | 100…200…400…600 | 469…481…523…574 |
| 35ХГСЛ | 100…200…300…400…500…600…700…800…900 | 496…504…512…533…554…584…622…693…689 |
| 35HML | 100…200…300…400…500…600…700…800…900 | 479…500…512…529…550…580…617…689…685 |
| 36Х18Н25С2 (4Х18Н25С2, ЭЯ3С) | 20 | 515 |
| 40 | 100…200…300…400…600 | 469…481…519…523…574 |
| 40G | 100…200…400…600 | 486…481…490…574 |
| 40L | 100…200…400…600 | 469…481…523…574 |
| 40Х10С2М (4Х10С2М, EI107) | 300…400…500 | 532…561…586 |
| 40Х13 (4Х13) | 20…100…200…300…400…500…600…700…800 | 452…477…502…528…553…578…620…666…691 |
| 40HL | 100…200…300…400…500…600…700…800…900 | 491…508…525…538…569…588…626…701…689 |
| 45 | 100…200…400…500 | 469…482…524…574 |
| 45G2 | 100…200 | 444…427 |
| 45L | 100…200…400…600 | 469…481…523…569 |
| 45Х14Н14В2М (ЭИ69) | 300…400…500…600 | 507…511…523…528 |
| 50 | 300…400…500 | 561…641…787 |
| 50G | 20…100…200…300…400…500…600…700 | 487…500…517…533…559…584…609…676 |
| 50L | 100…200…400…600 | 478…511…511…569 |
| 55 | 100…200…400…500 | 477…486…523…569 |
| 60 | 100…200…400…600 | 481…486…528…565 |
| ХН35ВТ (ЭИ612) | 100…200…300…400…500…600 | 511…544…569…590…595…595 |
| KHN64VMKYUTL (ZMI3) | 20…100…200…300…400…500…600… 700…800…900…1000 | 430…450…470…490…515…540…565… 590…625…650…1008 |
| KHN65VKMBYUTL (EI539LMU) | 20…100…200…300…400…500…600… 700…800…900…1000 | 424…436…480…493…505…518…548… 596…650…692…710 |
| KHN65VMTYUL (EI893L) | 20…100…200…300…400…500…600…700…800 | 425…430…440…470…500…510…550…615…650 |
| KHN65KMVYUTL (ZhS6K) | 20…100…200…300…400…500…600…700…800…900 | 380…400…420…445…470…485…515…560…610…660 |
| ХН70БDT (EK59) | 100…200…300…400 | 450…475…500…505 |
| KHN70KVMYUTL (TsNK17P) | 20 | 440 |
| KhN80TBYuA (EI607A) | 100…200…300…400…500…600 | 494…547…607…678…749…829 |
| Х15Н60-Н | 20 | 460 |
| Х20Н80-Н | 20 | 460 |
| Х23У5Т | 20…800 | 480…750 |
| Х27У5Т | 20…800 | 500…690 |
| A12 | 100…300…400…600 | 469…477…515…569 |
| R6M5 | 100…200…300…400…500…600…700 | 440…470…500…550…580…670…900 |
| P18 | 100…200…300…400…500…600…700 | 420…450…470…510…550…610…690 |
| U8, U8A | 20…100…200…300…400…500…600…700…800…900 | 477…511…528…548…565…594…624…724…724…703 |
| U12, U12A | 20…100…200…300…400…500…600…700…800…900 | 469…503…519…536…553…720…611…712…703…699 |
Average specific heat capacity of high alloy steels
The table shows the mass specific heat capacity of high-alloy steels with special properties, such as G13 steel and R18 steel. The heat capacity of G13 and R18 steels is given in kJ/(kg deg) at temperatures of 50...1300°C.
Average specific heat capacity of low-alloy steels
The table shows the mass specific heat capacity of low-alloy steels. The heat capacity values are given for the following steel grades: steel 30Х, 30Н3, 30ХН3, 30Г2, 50С2Г. The specific heat capacity of steels in the table is expressed in kJ/(kg deg) and is indicated depending on the temperature - in the range from 50 to 1300°C.
Specific heat capacity of carbon steels and cast iron at different temperatures
The table shows the values of the specific (mass) heat capacity of the following carbon steels and cast iron: steel 08, art. 20, art. 35, art. U8, electrical sheet steel, white cast iron, SCh10 cast iron. The heat capacity is presented in the table in the temperature range from 80 to 1573 K in the dimension kJ/(kg deg).
Specific heat capacity of alloy steels at different temperatures
The table shows the values of the mass specific heat capacity of steel of the following grades: steel 15L, 25L, 45L, 55L, 13N2ХА, R18, 11R3AM3F2, R6M5, 4Х13, 1Х12В2МФ, Х5М, 30ХМ, 30ХМА, 30ХГС, 30ХГСА, 1Х11МФ, 1Х12В IMF, 25Х2МФА, ХН35ВТ ( EI612, EI612K), Kh17N13M2T (EI448), Kh16N25M6 (EI395), Kh22N26, VZh100, ShH15. The mass heat capacity of alloy steels in the table is expressed in kJ/(kg deg) depending on temperature - in the range from 300 to 1400K.
Average specific heat capacity of carbon steels
The table shows the values of the mass heat capacity of iron and the following carbon steels: steel 08KP, st. 08, steel 20, 40, steel U8, U8′, u12. The mass specific heat capacity of carbon steels in the table is given in the dimension kJ/(kg deg) in the temperature range from 50 to 1300°C.
Sources:
Baths and batteries physics
The principles for calculating the heat capacity of metal utensils are applicable to batteries and bathtubs.
A cast iron battery takes longer to cool down.
Let me draw your attention once again to the fact that the rate of cooling of an object directly depends on the mass and specific heat capacity of the material from which it is made. Do not confuse heat capacity and thermal conductivity!
A cast iron battery is three times heavier than an aluminum one. Consequently, it has a 2.5 times greater heat capacity.
The question is often asked: why do cast iron batteries take longer to cool down than steel ones?
And the specific heat capacities - 540 J/(kg*K) for cast iron and 460 J/(kg*K) for steel - differ relatively little (15%). And the whole secret - to a large extent - lies in the significantly greater mass of cast iron batteries.
Battery section weight:
| Metal sections | Section weight, kg |
| aluminum | 0,5 — 1,5 |
| bimetal (steel with aluminum) | 1,5 |
| cast iron | 3,7 — 5,9 |
If we compare two batteries of equal weight - made of steel and cast iron - then at the same heating temperature, a cast-iron battery will retain heat by 15% more.
A cast iron bath retains heat.
Cast iron bath:
| Weight | 100 kg |
| Specific heat capacity coefficient of cast iron | 540 J/(kg*K) |
| Heat capacity of the cast iron bath itself | 100 kg * 540 J/(kg*K) = 54 kJ/K |
Steel bath:
| Weight | 30 kg |
| Specific heat capacity coefficient of steel | 720 J/(kg*K) |
| Heat capacity of the steel bath itself | 30 kg * 720 J/(kg*K) = 21.6 kJ/K |
That is, the amount of heat released when cooling by 1 degree for a cast iron bathtub is 2.5 times greater than for a steel bathtub (in our example).
Heat capacity of bath water:
| Volume | 100 liters = 0.1 cubic meters m |
| Density of water | 1000 kg/cu.m. m |
| Specific heat capacity coefficient of water | 4183 J/(kg*K) |
| Heat capacity of bath water | 0.1 cu. m * 1000 kg/cu.m. m * 4183 J/(kg*K) = 418.3 kJ/K |
This means that the temperature of hot water (40 degrees) poured into a bathtub at room temperature (20 degrees) will drop by 1 degree for a steel bathtub and by 2.5 degrees for a cast iron bathtub.
Similar articles:
Cast iron material: basic properties and important characteristics
Cast iron consists of carbon, iron and some impurities. It is one of the main materials of ferrous metallurgy. Cast iron is used in the manufacture of household and utility items, machine parts and in other industries. It is used in production, focusing and taking into account its properties and characteristics.
This article is precisely intended to tell you about the density of high-strength, liquid, white and gray cast iron, its melting points and specific heat capacity will also be considered separately.
Cast iron, like any metal, has the following properties: thermal, physical, mechanical, hydrodynamic, electrical, technological, chemical. Let's look at each properties in more detail.
This video talks about the structure and composition of cast iron alloys and the dependence of their properties on a specific composition:
Heat capacity
The thermal capacity of cast iron is determined using the displacement rule. When the heat capacity of cast iron reaches a temperature period, the beginning of which begins at a temperature whose value is greater than phase transformations and ends at a level equal to the melting temperature, then the heat capacity of cast iron takes on a value of 0.18 cal/Ho C.
If the value of the melting temperature exceeds the absolute value, then the heat capacity is equal to 0.23 ± 0.03 cal/Ho C. If the solidification process occurs, then the thermal effect is equal to 55 ± 5 cal. The thermal effect depends on the amount of pearlite when pearlite transformation occurs. Typically it takes a value of 21.5 ± 1.5 cal/G.
The volumetric heat capacity is taken to be the product of the specific gravity and the specific heat capacity. For solid cast iron this value is 1 cal/cm3*ºС, for liquid cast iron – 1.5 cal/cm3*ºС.
The specific heat capacity of cast iron is 540 J/kg C.
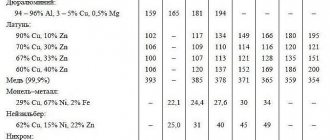
Specific heat capacity of cast iron and other metals in table form
Thermal conductivity
Unlike heat capacity, thermal conductivity is not determined by the displacement rule. Only if the amount of graphitization changes, the composition of the cast iron will affect the thermal conductivity.
Thermal diffusivity
The thermal diffusivity value of solid cast iron (for large calculations) can be taken equal to its thermal conductivity, and that of liquid cast iron – 0.03 cm2*/sec.
Read below about the melting point of cast iron.
Melting temperature
Cast iron melts at a temperature of 1200ºС. This temperature value is 300 degrees lower than the melting point of steel. With an increased carbon content, this chemical element has a close connection at the molecular level with iron atoms.
During the process of melting cast iron and its crystallization, the carbon component cannot completely penetrate the structural lattice of iron. As a result, the material cast iron takes on the property of brittleness. Cast iron is used for parts that require increased strength. However, cast iron is not used in the manufacture of objects that will be subject to constant dynamic loads.
The table below shows the melting point of cast iron in comparison with other metals.
Melting point of cast iron and other metals
Weight
The weight of the material varies depending on the amount of fixed carbon and the presence of a certain percentage of porosity. The specific gravity of cast iron at the melting point can be significantly reduced depending on the presence of impurities in the cast iron.
In addition, the linear expansion of the metal and the structure of cast iron changes depending on the state of each indicator. That is, these are dependent quantities.
The specific gravity of each cast iron differs depending on the type of material. Gray cast iron has a specific gravity of 7.1±0.2 g/cm3, white cast iron has a specific gravity of 7.5±0.2 g/cm3, and malleable cast iron has a specific gravity of 7.3±0.2 g/cm3.
The video below will tell you about some of the physical properties of cast iron:
The volume of cast iron, passing through the temperature of phase transformations, reaches an increase of 30%. However, when heated to 500ºC, the volume increases by 3%. Growth is aided by graphite-forming elements. Carbide-forming components inhibit volume growth. The same growth is prevented by applying galvanic coatings to the surface.
carbon is usually at least 2.14%. Thanks to the carbon content, cast iron has excellent hardness. However, the plasticity and malleability of the material suffers against this background.
We will talk about the density of cast iron below.
Density
The density of the described material, cast iron, is 7.2 g/cm3. If we compare other metals and alloys with cast iron, this density value is quite high.
Due to its good density, cast iron is widely used for casting various parts in industry. In terms of this property, cast iron is only slightly inferior to some steels.
Tensile strength
The compressive strength of cast iron depends on the structure of the material itself. The components of the structure gain their strength along with an increase in the level of dispersion.
The tensile strength is strongly influenced by the number, size, distribution and formagraphite inclusions. The tensile strength decreases by a noticeable amount if the graphite inclusions are arranged in the form of a chain.
This arrangement reduces the cohesion of the metal mass.
The tensile strength reaches its maximum value when the graphite takes on a spheroidal shape. This form is obtained without the influence of temperature, but when cerium and magnesium are included in the cast iron mass.
- When the melting temperature increases to 400ºС, the tensile strength does not change.
- If the temperature rises above this value, the tensile strength decreases.
- Note that at temperatures from 100 to 200ºС, the tensile strength can decrease by 10-15%.
Plastic
The ductility of cast iron largely depends on the shape of the graphite, and also depends on the structure of the metal mass. If graphite inclusions have a spheroidal shape, then the percentage of elongation can reach 30.
- In ordinary gray cast iron, the elongation reaches only a tenth.
- In annealed gray cast iron, the elongation is 1.5%.
Elasticity
Elasticity depends on the shape of the graphite. If the graphite inclusions did not change, and the temperature increased, then the elasticity remains at the same value.
The elastic modulus is considered a conditional value, since it has a relative value and directly depends on the presence of graphite inclusions. The elastic modulus decreases if the number of graphite inclusions increases. Also, the elastic modulus increases if the shape of the inclusions is distant from the globular shape.
Impact strength
This indicator reflects the dynamic properties of the material. The impact strength of cast iron increases:
- when the shape of graphite inclusions is close to spherical;
- when the ferrite content increases;
- when the graphite content decreases.
Endurance limit
The endurance limit of cast iron becomes greater when the frequency of loading increases and the tensile strength becomes greater.
Dynamic viscosity
Viscosity becomes less if the amount of manganese in cast iron increases. A decrease in viscosity was also noticed with a decrease in the content of sulfur impurities and other non-metallic components.
The process is affected by the temperature value. Thus, the viscosity becomes less when the ratio of two temperatures is directly proportional (the temperature of the experiment and the start of solidification).
Surface tension
This figure is 900±100 dynes/cm2. The value increases as the amount of carbon decreases and undergoes significant changes in the presence of non-metallic components.
Toxicity
Cookware is often made from cast iron. The fact is that cast iron as a material is non-toxic and tolerates temperature changes well.
Electrical characteristics
The electrical conductivity of cast iron is assessed using Kurnakov's law. The electrical resistance of some types is given below:
- white cast iron - 70±20 Mk·oi·cm.
- gray cast iron - 80±40 Mk·oi·cm.
- malleable cast iron - 50±20 Mk·oi·cm.
According to the weakening effect on electrical resistance, the elements of solid cast iron can be arranged as follows: first - silicon, second - manganese, third - chromium, fourth - nickel, fifth - cobalt.
Technological features
Fluidity can be determined by various methods. This indicator depends on the shape and properties of cast iron.
Fluidity becomes greater when:
- overheating increases;
- viscosity decreases;
- hardening becomes less.
Fluidity also depends on the heat of fusion and heat capacity.
Chemical properties
The corrosion resistance of a material depends on the external environment and its structure. If we consider cast iron from the side of decreasing electrode potential, then its components have the following arrangement: graphite-cementite, phosphide eutectic-ferrite.
It should be noted that the potential difference between graphite and ferrite is 0.56 V. If the dispersion increases, the corrosion resistance becomes less. With a strong decrease in dispersion, the opposite effect occurs, and corrosion resistance decreases. Alloying elements also affect the resistance of cast iron.
Industrial cast iron contains impurities. These impurities greatly affect the properties, characteristics and structure of cast iron.
- Thus, manganese inhibits the graphitization process. The release of graphite is stopped, as a result, cast iron acquires the ability to bleach.
- Sulfur degrades casting and mechanical properties.
- Sulfides are mainly formed in gray cast iron.
- Phosphorus improves casting properties, increases wear resistance and increases hardness. However, against this background, cast iron still remains fragile.
- Silicon has the greatest influence on the structure of the material. Depending on the amount of flint, white and ferritic cast iron are obtained.
To obtain certain characteristics, special impurities are often introduced into cast iron during its manufacture. Such materials are called alloy cast iron. Depending on the added element, cast iron can be called aluminum, chromium, or sulfur. Basically, elements are introduced with the aim of obtaining a wear-resistant, heat-resistant, non-magnetic and corrosion-resistant material.
This video will compare the properties of cast iron and steel:
Thermal conductivity.
Thermal conductivity is numerically equal to the amount of heat (J) passing through a unit area (sq.m) per unit time (sec) with a unit temperature gradient.
Thermal conductivity coefficients from the reference book:
| Metal | Thermal conductivity coefficient, W/(m*K) |
| Copper | 390 |
| Aluminum | 236 |
| Steel | 47 |
| Cast iron | 42 |
Conclusion: Cast iron distributes heat slowly. In other words, meat in a cast iron frying pan will not burn (among other things) due to a more even distribution of heat.
The situation is similar in cooking barbecue outdoors. Cooking meat over coals allows you to bake the pieces. Cooking over an open fire simply cooks the outside of the meat, leaving the inside raw.