To give metal surfaces a shine and improve their appearance, they are chrome-plated. This technology is most popular in the automotive industry. It is necessary not only to improve the appearance, but also to increase the strength of the workpiece. Among the most effective processing technologies, experts highlight galvanic chrome plating.
Galvanic chrome plating
The essence of the process
Chrome plating is a technological process of metalizing products with chromium. Thanks to it, the technical characteristics of the material are improved and the surface layers are strengthened.
The main advantages of the procedure:
- The base is restored and becomes stronger. The wear resistance indicator increases.
- Cracks not exceeding 1 mm in thickness are closed.
- Dirt and dust stick to the chrome surface worse.
- The decorative qualities of processed parts are improved.
The product is immersed in a chromic acid bath through which voltage is passed. The workpiece being processed acts as a cathode. Passive anodes are the walls of the container and additional plates, which are made from an alloy of antimony and tin.
Many novice car enthusiasts compare chrome plating with nickel plating, try to find the differences, and get confused about the advantages and disadvantages. Nickel-based plating is only superior in appearance. Its strength and wear resistance indicators are much worse.
Chrome disk (Photo: Instagram / funchrome)
The process of chrome plating parts
The methods of applying a layer of chromium to the surface of the metallized part differ in the methods of setting (holding) with each other. They can be classified as follows:
- adhesive setting (due to mechanical action);
- due to metallic bonds: diffusion zone within the border of two surfaces;
- diffusion zone of the entire covering layer.
Chrome plating technology involves several stages:
- preparatory;
- application process;
- final.
Preparatory stage. At this stage, those types of work are performed that will allow the chromium layer to be securely fixed and retained on the surface for a long time. Before chrome plating, products are ground and, if necessary, polished. After the finishing operation, the products are washed, dried and wiped with a soft material. Those surfaces (holes, internal cavities) that are not subject to metallization are insulated. The parts are installed (hung) on a device that is designed to introduce parts into the processing zone. A mandatory degreasing process is carried out. Pickling is carried out to increase the ability of adhesion.
The process of applying chromium to a surface. The technology of chrome plating of parts, depending on the application method, occurs in three types:
- in a cold state;
- in a heated state;
- diffusion.
For example, during the electrolytic method, products are placed in a bath with an electrolyte solution. The operating temperature of the electrolyte depends on its composition. The specified temperature must be maintained throughout the entire process, which guarantees a uniform structure of the applied layer and uniform thickness.
The metallized products act as an anode. The duration of the chrome plating process directly depends on the required coating thickness.
Decorative chrome plating of a part
After applying chrome, the products are dried. If drying is carried out in a drying cabinet, its duration will be 5-10 minutes at a temperature of 85°C-100°C. If drying is carried out by blowing with compressed air, then its duration will be 0.5-3 minutes at a temperature of 18°C-25°C.
To increase the strength and hardness of the coated layer, it is subjected to heat treatment. The duration of aging in the oven is several hours at a temperature of about 200°C. The thickness of the coating applied to steel ranges from 0.003 mm to 0.025 mm. If you use a change in current polarity (reverse), then the thickness of the chrome plating is adjusted to 0.03 mm.
Classification
Chromium plating can be done in several ways:
- Galvanic. There are two types - diffuse, electrolytic. The first option involves applying chrome using a galvanic brush. The electrolytic type involves the use of a chromic anhydride solution into which the workpiece is immersed.
- Vacuum. The workpieces are placed in a vacuum chamber into which chromium vapor is supplied, which deposits on the metal surfaces, creating a protective layer.
- Chemical. The technology does not require the use of electric current. The mixture for treatment is prepared from sodium citrate, phosphorus, sodium hydroxide, and glacial acetic acid.
- Catalytic method. It can be classified as a chemical treatment. A working composition that does not contain acids is applied to the surface of the parts. It consists of silver in an alkaline ammonia solution. Additionally, a reducing agent is used - hydrazine or formalin.
- Thermochrome plating. The products are heated and coated with a working mixture - chromium powder or ferrochrome.
The difference between pseudo-chrome plating and real chrome plating
The simplest method of chrome plating, which cannot be considered full metallization, involves the use of paint containing at least 75% chrome dust. Metal-containing paint is applied to the surface of a cold product (for this, use a spray gun or a regular paint brush). Thus, a kind of barrier is created that protects the metal from corrosion. The result of such chrome plating, if done correctly, can last at least five years. However, if the coating is damaged, corrosion will affect not only the surface of the metal, but also its deep layers.
Truly effective chemical metallization involves the use of:
- a special container in which galvanic processes take place;
- electrolyte for chrome plating;
- source of constant electric current.
This set of accessories and consumables is the minimum for chrome plating.
The complete set of chrome plating equipment also includes degreasing and rinsing baths, an oil separator and drying chambers
Necessary equipment
Tools and equipment:
- DC source with adjustable output voltage. It is permissible to process small parts when using a charger for mobile phones.
- Galvanic bath. Must be made of heat-resistant plastic or glass. The main condition is resistance to high temperatures.
- Thermometer - necessary to control the temperature during the work process.
- A heating element. The best option is a ceramic heating element. The heater must withstand prolonged exposure to acids.
For processing, it is necessary to install at least two galvanic baths so as not to constantly change the reagents in one container.
Galvanic bath (Photo: Instagram / galvaprom)
Mini galvanizing line for nickel plating and chrome plating
Products / Galvanic equipment for electroplating production / Mini galvanizing line for nickel plating and chrome platingPlease note that preparing a preliminary project for organizing a galvanic section costs RUB 49,000. It includes the selection of equipment according to a given performance, a price review of each piece of equipment, the development of a route map for the movement of workpieces, operating modes, the chemical composition of working solutions, a general scheme for wastewater treatment if necessary, a description of possible errors in the application of galvanic coatings and methods for solving them, etc. .d. This preliminary draft is for informational and advisory purposes only and serves as the basis for a detailed financial assessment of the planned production and is not a detailed instruction for production. Based on these materials, you receive an estimated cost of the main and additional equipment and have the opportunity to evaluate the actual investment. If necessary, you will subsequently be able to independently operate with the received data. All this information is presented in an accessible, easy-to-understand form, without detailed technical calculations and calculations that are not included in this work.
Mini line for galvanizing, nickel plating, chrome plating, bluing, etc., design and manufacturing - this is the list of services provided by our company. If you need to make a mini-line for applying coatings such as zinc, nickel, chromium, copper, tin-bismuth alloy and other protective coatings, and the amount of work is not too large, then a mini galvanic line is your choice.
Galvanizing line, nickel plating, chrome plating, their cost depends directly on several main factors, we list them:
1. Overall dimensions of the workpieces - the internal dimensions of the galvanic baths, which will be the main element of the designed mini-line, depend on this.
2. The total surface area of the workpieces - the type and power of galvanic rectifiers will depend on this.
3. The type of galvanic coating applied, namely the chemical composition and temperature of the working processes - the material (as a rule, in the vast majority of cases polypropylene is used for the manufacture of galvanic baths) from which the baths will be made depends. Availability of on-board suction.
4. The number of galvanic baths included in the mini-lines for galvanizing, nickel plating, chrome plating, copper plating, bluing differ from each other due to the peculiarities of the technological process of preparation, galvanization of the workpieces themselves, and then wastewater disposal.
5. The need to equip the galvanizing, nickel plating, chrome plating line with heating elements: heating elements made of stainless steel, titanium, zirconium, as well as fluoroplastic heaters FEN, control systems and monitoring of operating temperatures of chemical solutions.
6. The need to use additional means of process automation, namely, equipping mini-lines with galvanic drums and installations for filtering galvanic solutions.
Here are the main factors influencing the final cost when calculating a line for galvanizing, nickel plating, chrome plating, copper plating, bluing, etc. To calculate the cost of a galvanic mini line, you need to send an application to or call us at (383) 219-50-92 (88) .
Please note that we can produce a universal mini-galvanic line for applying various galvanic coatings by changing technological solutions. For chrome plating you will need a separate fluoroplastic bath, because... In this process, high temperature plus a chemically aggressive solution, plus a more powerful galvanic rectifier.
An example of the organization (scheme) of a site for nickel plating of metal products with an intermediate copper sublayer with collection and screening baths:
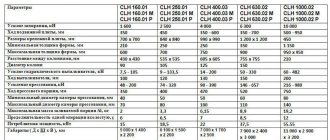
Maximum workpiece size 1000*600*40 mm (clothes dryer)
Internal dimensions of galvanic baths with side suction: 1460*660*800 mm – 15 pcs.
Galvanic rectifiers – 4 pcs.
Electric heating system and control of working temperature of solutions – 1 pc.
Installation of filtration of electrolytes, solutions – 1 pc.
Pump for pumping chemically aggressive media – 1 pc.
Chemical resistant hose – 20 m
* Bathtub material - polypropylene, operating temperature from -30 to +85 degrees Celsius
The price for a nickel plating line in this configuration is RUB 1,600,000. with VAT.* without delivery, installation and commissioning.
*The price indicated is approximate. The actual price of the line depends on the configuration and size of the baths, as well as the availability of additional equipment.
A galvanizing line with the specified bath sizes will be 30% cheaper
Production time 35 - 50 working hours. days.
How to properly prepare the product for the procedure?
The quality of galvanic chrome plating depends on the preparation of the working surfaces. Stages:
- Cleaning from dirt, plaque, rust. For this, sandpaper and angle grinders are used.
- Degreasing surfaces with calcined water. To make the product, you need to mix 1000 ml of plain water with 50 ml of calcined water. Add 5 g of silicate glue and 0.15 kg of sodium hydroxide to the finished liquid. Mix thoroughly and heat the mixture.
The parts are immersed in degreasing liquid for 20 minutes.
Carrying out
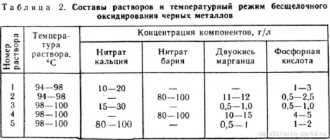
Chrome plating at home must be carried out in compliance with a number of rules and safety precautions. Initially, it is necessary to prepare the equipment, make a working mixture - electrolyte. Manufacturing instructions:
- Buy a large container of distilled water. Pour the required amount of liquid into a container for heating. Preheat to 60°C.
- Add 250 g of chromic anhydride (per 1 liter of water). To stir thoroughly.
- Pour the liquid into the galvanic bath.
- Add 2.5 grams of sulfuric acid.
To equalize the density of the electrolyte, it is necessary to pass the rated current through it and keep the liquid in a dark room for 24 hours.
For galvanic chrome plating, 3 elements are required:
- Anode - this element is the workpiece being processed. The anode should not be allowed to touch the cathode.
- Cathode - made of tin with lead or pure lead. It is a metal plate whose area should exceed the anode.
- Electrolyte is the working composition, without which it is impossible to carry out chrome plating.
Chrome plating process:
- Warm up the electrolyte to 52°C.
- Place the workpiece in a galvanic bath.
- Apply operating voltage. The part must be processed for up to 1 hour. The more complex the shape, the longer the processing takes.
After the work process, the part is dried for 3 hours. You must not touch it with your hands.
Galvanic chrome plating procedure (Photo: Instagram / galvaprom)
Types of chrome plating
According to the classification, the metallization process occurring due to mechanical adhesion belongs to the first group, and due to atomic mechanical bonds - to the second group. The second group is divided into two subgroups: 2a - border diffusion; 2b – total diffusion.
Group 1 includes the following chrome plating methods:
- electrical coating;
- electric arc or gas flame spraying (pulverization);
- chemical application;
- vacuum application in a cold environment.
Result of chrome plating
Group 2 includes:
2a:
- plasma spraying;
- electrophoresis;
- vacuum application in a heated environment;
- electrical coating followed by annealing;
- deposition of pure metal from carbonate compounds in a gaseous environment;
2b:
- diffusion application of elements.
Hard chrome plating
Hard chrome plating has found wide application in the manufacture of parts subject to high wear, active corrosion in aggressive environments, in the restoration of metal parts, to increase the service life of tools (cutting, measuring), as well as for decorative finishing of products made from non-metallic materials.
Hard chrome plating is carried out using the following methods:
- galvanic (described above);
- catalytic, in which chromium is reduced on the surface from ammonia and silver salts;
- vacuum, in which the reagent applied to the surface being treated exhibits diffusion activity under negative pressure;
- thermochemical, which can be compared to the cementation of products.
Using the thermochemical method, chrome plating is carried out in a carburizer consisting of crushed chromium and kaolin in a proportion of 55-45%. To prevent oxidation of chromium at high temperatures, hydrogen is blown through the boxes with parts and carburizer. The duration of chrome plating is three hours. During this time, the layer thickness reaches 0.15 mm at a temperature of 1300°C, and 0.8 mm at a temperature of 1400°C.
Chrome plating by electrolysis
Chrome plating by electrolysis involves the easy removal of hydrogen compared to chromium from the electrolyte. The electrolyte is chromic acid. The baths are equipped with insoluble lead anodes.
A sulfate electrolyte based on chromic anhydride with sulfuric acid CrO3:H2SO4 is widely used.
The concentration of the solution is selected based on the nature of the coating and the complexity of the part’s shape.
At a low metallization temperature (not higher than 35°C), the chrome surface has a matte gray tint. The intensity and current density does not affect the process. When the temperature increases to 65°C and the current density, the surface becomes shiny. With a further increase in temperature and current density (up to 30 A/dm2), chromium has a milky tint.
Also, the quality of the coated surface depends on the electrolyte concentration. The chrome coating obtained using a concentration of up to 150 g/l is characterized by high hardness and wear resistance. Highly concentrated electrolytes, up to 450 g/l, are used for decorative coatings.
Galvanic chrome plating
Galvanic chrome plating is the most common modern method of chrome plating. It is carried out in two ways: in an electrolyte environment and by diffusion. The electrolytic method is similar to chrome plating by electrolysis; they differ only in the modes of the process.
The diffusion method is the process of saturating a surface under certain conditions from applied reagents. Finished parts have: strength and hardness, viscosity and elasticity, wear, heat and corrosion resistance.
Safety precautions
In order not to harm your body, you should follow safety precautions:
- Before chrome plating, you need to establish ventilation and remove flammable mixtures from the work area.
- When preparing electrolyte and the work process, use protective gloves, goggles, and a respirator.
- Buy special bags for chemical waste in which you need to pack the remaining waste after processing.
- The room must be cleared of any organic matter. Otherwise, the items will be damaged.
Doctors recommend lubricating the inside of the nose with lanolin and Vaseline. The components are mixed in a 1:2 ratio. This will help protect the body from exposure to harmful fumes.
Galvanic chrome plating is used in various industries. With its help, the appearance of products is restored and their technical characteristics are improved. The treatment can be done at home, but it is important to mix the chemical components correctly.