The company provides chrome plating services for parts made of all grades of steel, aluminum alloys and titanium. Maybe chrome plating parts up to 1000 mm long. and weighing up to 50 kg.
The thickness of the chrome coating is from 1 micron. The cost of chrome plating is from 150 rubles. for 1 dm2. To place an order for chrome plating, you must send us product drawings and quantities. The cost of applying chromium is calculated based on the surface area of the parts being processed, as well as the thickness of the coating. You can evaluate the quality of chrome plating by ordering the processing of a trial batch of products.
- Processed materials: steel of any grade, aluminum alloys, titanium.
- Overall dimensions of products (LxWxH): 1000mm.x500mm.x500mm.
- Requirements for the metal surface: clean without traces of rust and scale.
- Chrome plating price: calculated individually, from 150 rubles. for 1 dm2.
Chrome plating in theory and practice
| Chrome is a silvery-white metal with a bluish tint. The atomic mass of chromium is 52.0, valence is 2, 3, 6, density is 7.1 g/cm3, melting point is 18900C. The hardness of the chromium coating varies from 3 to 18 GPa and depends on the composition of the electrolyte and the process mode. |
Chrome plating occupies a special place among electroplating coatings and is used in many areas. The advantages of chrome plating include high hardness of the coating (on average higher than that of hardened steel), resistance of chrome-plated parts to corrosion and aggressive environments, heat resistance, and beautiful appearance.
Chrome car part
Depending on their purpose, chrome coatings are divided into decorative and functional. Decorative coatings are applied in the form of a thin (less than 1 micron) layer on a copper or nickel sublayer. Products treated in this way, in addition to their attractive appearance (shiny light metallic), become resistant to corrosion. Functional coatings are applied directly to the metal; the thickness of such coatings can reach several millimeters. The practical application of functional chrome plating is the coating of tools, templates, molds, repair of worn parts, reduction of friction of mating parts, etc. Chrome is stable in a humid atmosphere, in hydrogen sulfide, alkali solutions, nitric acid and organic acids. In the atmosphere, due to its strong passivation ability, chromium retains its color and shine for a long time. There are black chrome coatings, which are mainly used to give products protective and decorative properties.
Structure and composition of chrome coatings.
By changing the electrolysis mode, it is possible to obtain different types of chromium deposits, differing in their properties and, therefore, in their scope of application. The greatest technical and economic effect is achieved when using wear-resistant and corrosion-resistant chromium plating. It is important to note that hardness does not always correlate with wear resistance.
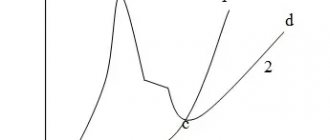
The diagrams in Figure 2 clearly demonstrate the areas of obtaining hard and wear-resistant coatings under standard chromium plating modes in “universal” sulfate electrolytes.
Figure 2 - Diagrams of conditions for obtaining hard (T) and wear-resistant (I) chromium coatings under standard chromium plating modes in “universal” sulfate electrolytes: in a diluted electrolyte (a) and a standard electrolyte (b).
The wear resistance of coatings obtained from a “universal” standard electrolyte increases with increasing temperature and, having passed through a maximum at 55-65 ° C, decreases to a minimum at 75 ° C. For deposits obtained from a dilute electrolyte, the maximum wear resistance shifts to the region of higher temperatures The ductility of electrolytic chromium also significantly depends on the chromium plating mode. Brittle chromium deposits (shiny and matte) are obtained at low electrolyte temperatures and high current densities, while more plastic coatings are obtained at high temperatures and low current densities (milky deposits).
Electrolytic chromium deposits have an extremely fine crystalline structure (Figure 3).
Figure 3 - Microimage (from left to right) of hard, thin shiny and milky crack-free chrome, x100.
The smallest sizes (0.001-0.01 microns) are crystals of shiny chromium. The sizes of matte and milky chromium crystals are 0.1-10 microns.
Below are photographs of microsections of a cross section of a chromium layer at 1000x magnification (Figure 4).
a b
Figure 4 - Microphotographs of transverse sections of matte (a) and shiny chrome (b).
Electrolytically deposited chromium contains oxygen, hydrogen and trace amounts of nitrogen. The mass fraction of oxygen is 0.2-0.5 mass fractions, hydrogen - 0.03-0.07 mass fractions. The volume of gases included in the deposit depends on temperature and current density: with increasing temperature and decreasing current density, the volume of gases in the deposit decreases slightly.
Two main structural modifications of electrolytically deposited chromium are known:
- α-Cr with a cubic body-centered crystal lattice and a density of 7.1 g/cm3
- β-Cr with a close-packed hexagonal crystal lattice and a density of 6.08 g/cm3.
At high current densities and elevated electrolyte temperatures, a predominantly cubic α-Cr structure is formed; at low current densities and room temperature, a predominantly hexagonal β-Cr structure is formed. This structure is stable only at temperatures below 25° C, and at higher temperatures it transforms into a stable α-Cr modification.
Chromium deposits are characterized by high internal stresses, the cause of which is the spontaneous transition of unstable β-Cr into the stable modification α-Cr, which has a higher density, as a result of which a network of microcracks is formed in the chromium deposits. As the electrolyte temperature increases, internal stresses and the number of cracks decrease.
All chromium coatings are characterized by high hardness, but the hardness of cubic chromium is significantly higher than the hardness of hexagonal chromium. Hardness is determined by the electrolysis mode, as mentioned above.
Features of the chrome plating process
The chrome plating process has a number of distinctive features:
- The electrolytes used in chrome plating have an extremely low dissipative ability, and therefore chromium is deposited on the recessed surfaces of products (holes, grooves, grooves) much more slowly.
- The concentration of current on the protruding elements of products leads to the deposition of a thicker layer of chromium on them. To reduce the uneven distribution of current density in such areas, aluminum or lead foil or wire is used.
- During the chrome plating process, it is necessary to strictly observe the technological regime - electrolyte temperature and current density.
- Before the chrome plating process, products made of carbon steel are subjected to anodic pickling in a chrome electrolyte for 3-5 minutes.
- The beginning of the chrome plating process (approximately 1-2 minutes) must be carried out at twice the current density.
- Chromium is not deposited on the surface of nitrided steel. Before chrome plating, it is necessary to remove the nitrided layer.
- Products after polishing or pre-nickel plated products must be chrome plated immediately. Otherwise, surface treatment (glazing) with aluminum oxide is required.
- Products made of copper and brass must be heated in hot water before immersing in an electrolyte solution. Immersion in the electrolyte is carried out under current.
- Products after electropolishing are pre-etched in a solution of hydrochloric acid.
Like other types of coatings, chrome plating requires preliminary surface preparation. In addition to the obvious need to clean the surface of the product from dirt, lubricant residues, slag, etc., before chrome plating, the part is subjected to mechanical processing to obtain a certain class of surface cleanliness. Shiny chrome plating requires a cleanliness class of at least 5; for ordinary chrome plating, class 3 is sufficient; copper and brass products must have class 4.
More information on preparing the surface of a product for galvanic treatment, surface roughness and cleanliness classes can be found in the article.
Individual areas of the product that do not require chrome plating are isolated using tsapon varnish (a solution of celluloid in acetone). The varnish is applied with a brush in several layers, each layer is dried before applying the next one. After coating, the insulation layer is removed mechanically.
Composition of electrolytes and chrome plating modes
| Electrolyte composition and operating mode | Electrolyte A | Electrolyte B | Electrolyte C |
| Chromic anhydride, g/l | 150 | 250 | 350 |
| Sulfuric acid, g/l | 1,5 | 2,5 | 3,5 |
| Chrome plating temperature, 0C | 55-60 | 45-55 | 35-45 |
| Cathode current density, a/dm2 | 45-100 | 15-60 | 10-30 |
| Voltage, in | 12 | 12 | 12 |
| Current output, % | 16-18 | 13-15 | 10-15 |
| Dispersive power | High | Average | Low |
As can be seen from the table, the electrolyte for chrome plating is a mixture of chromic anhydride and sulfuric acid. The sulfuric acid content is approximately 1% of the anhydride content.
- Electrolyte A is an electrolyte for hard chrome plating of simple shaped products. Electrolyte depletion occurs at a high rate. The sediments are thick with growths on the edges.
- Electrolyte B is an electrolyte for a wide range of shiny coatings, used for decorative, hard and porous chrome plating of steel, nickel, copper and other metals.
- Electrolyte C is a decorative coating electrolyte for copper and nickel with a low depletion rate.
The essence of galvanic chrome plating of metal
In most electroplating processes, the source of the plating metal is the anode. In contrast, during chromium plating, anions arise directly from the electrolyte, the basis of which is a solution of chromic acids formed when chromic anhydride is dissolved in water.
In this technology, the cathode is usually the workpiece, and the role of a non-consumable passive anode is played by plates or bath lining made of a metal electrolyte inert to acids.
Passive anodes in chrome plating are usually made from lead or its alloys (with tin and antimony). Chromic acid has strong corrosive properties, so acid-resistant materials are used in the production of chrome plating equipment.
The release of chromium anions in the electrolyte volume during the chrome plating process occurs unevenly, therefore, galvanic baths are equipped with special devices that ensure a constant supply of mixed electrolyte to the cathode zone (to the metal surface of the chrome-plated part).
In addition, due to the constant decrease in chromium, the electrolyte must be periodically regenerated by adding chromium anhydride and reagents consumed during the chromium plating process.
The surface appearance and mechanical properties of the chromium coating directly depend on the components of the electrolytic solution, the degree of its heating and current density.
By varying these indicators, it is possible to achieve different types of surface of chromed metal: from milky and matte to mirror-shiny, as well as a wide range of values of hardness, density and porosity of the deposited chromium.
Preparation and adjustment of electrolyte
Distilled water is used to prepare the electrolyte. The composition is prepared directly in a chrome plating bath - the calculated amount of chromic anhydride is dissolved, after which the amount of sulfuric acid entering the solution is measured (chromic anhydride has minor impurities of sulfuric acid). Based on the analysis results, the missing amount of sulfuric acid is added to the solution, and the solution is thoroughly mixed. Before starting the chrome plating process, the electrolyte undergoes current treatment using lead anodes and steel cathodes. The temperature of the preparation process is 45-600C, the current density is the same as during chrome plating. Preparation is carried out with the aim of accumulating 2-3% trivalent chromium in the galvanic bath, which requires from 2 to 6 hours. The content of trivalent chromium should not exceed the specified value, since otherwise the quality of the coating will decrease. Analysis of the electrolyte composition is usually carried out at least once a week. If necessary, it is adjusted. The content of chromic anhydride in the electrolyte is checked every shift. The specific gravity of the electrolyte is measured using a hydrometer, then the anhydride content is determined using a special table.
Let's take a closer look at the types of chrome coating.
Possible defects and their causes
Often, during metallization, an effect such as hydrogenation occurs - the hydrogen content in chrome-plated steel increases. Due to this problem, the strength and ductility of the metal are reduced due to changes in its crystal lattice. The reasons for hydrogenation of steel are varied, most often it is associated with an increase in temperature during the galvanization process.
Other troubles that can happen when chrome plating products:
- Uneven shine. Happens when the current supplied to the anode is high. There may be no shine at all if there is too little or too much chromic anhydride or too much sulfuric acid.
- Brown spots. If the part has such defects, the rate of anhydride in the solution is very high or there is not enough sulfuric acid.
- Softness of the coating. The reason is low current during galvanization or a decrease in water temperature.
- Fast chrome peeling. The reason is poor degreasing before work, a decrease in the temperature of the solution.
- Craters on the surface of the product. It happens due to the retention of hydrogen bubbles on oxidized, porous substrates.
Excellent results can only be obtained by strictly following the technology. This will give the desired effect, saving a significant amount of money.
Protective and decorative chrome plating
As stated earlier, protective and decorative chrome plating is intended to give products a beautiful appearance - a shiny metal surface and increase corrosion resistance.
Protective and decorative chrome plating is widely used in the automotive industry. It's hard to imagine a classic car or a classic chopper without chrome interior or exterior parts. In modern cars, the amount of chrome is much less, but almost all tuning workshops offer quite popular services for applying chrome coating to various elements of cars or motorcycles. Many workshops are engaged in chrome plating of car wheels, many offer services for restoring worn parts of cars and motorcycles, giving them their original beautiful appearance. If hard chrome plating is used, the service life of individual parts and assemblies can be significantly increased (the hardness of the chrome coating is much higher than the hardness of steel). It is quite common to apply chrome coatings chemically, but only with electrolytic chrome plating is it possible to obtain a coating that not only looks attractive, but also has increased resistance to aggressive environments (dirt, sand, reagents) and to constant mechanical stress.
Another area of application of decorative chrome plating is the manufacture of trade, advertising and exhibition equipment. An example is chrome plating of metal shopping baskets, trolleys or hangers - the products receive significantly higher performance and decorative characteristics compared to galvanized ones. The increased price compared to galvanized products (by about 30-50%) is offset by a significant increase in service life, and in the manufacture of exhibition equipment, an attractive, bright appearance often becomes the main criterion in choosing a coating.
To obtain a high-quality protective and decorative coating, copper and nickel are first deposited on steel, and only then chromium itself. Each layer must be polished before applying the next. A layer of copper is deposited until a coating with a thickness of 10-15 microns is obtained; in this case, it is necessary to provide an allowance for polishing with a thickness of approximately 3 microns; with a copper layer thickness above 15 microns, the allowance must be at least 7 microns. A nickel layer 15 microns thick should have an allowance of 2 to 5 microns. It is possible to apply chromium directly to the surface of the steel, and the layer thickness should be at least 40 microns.
Products made of non-ferrous metals - copper or brass - are coated with a layer of nickel before chrome plating. If the operation of such products does not involve intense mechanical impact on their surface, then it is possible to apply a layer of chromium directly to the metal.
Cathodic mechanical chrome plating (galvanic honing).
An analysis of modern literary sources covering the issues of intensification of chrome plating processes, as well as modern Russian chrome plating technologies, has shown that applying shiny chrome coatings to cylindrical parts or rod-type parts made from a standard sulfate electrolyte at current densities of 3000-6000 A/m2 and electrolyte temperatures of 45 -70 °C allows the technology of cathodic mechanical chromium plating (CMC) or galvanic honing. This technology was developed by specialists from the Federal State Unitary Enterprise “TsNIIM” (St. Petersburg).
KMH technology involves chrome plating of cylindrical parts with a simultaneous mechanical (abrasive) effect on the cathode surface, that is, combining the chrome plating process with honing or lapping the surface with special polishing elements. According to developers' estimates, the wear resistance of chrome coatings obtained using the CMC technology, compared with coatings obtained by standard chrome plating of cylindrical parts, increases by 2-4 times [4]. In addition, the use of cathodic mechanical chromium plating makes it possible to obtain thick-layer chrome coatings (thickness over 100 microns) with a roughness corresponding to high classes of surface finish (not lower than class 9) without intermediate mechanical treatment.
The essence of the galvanic honing process is a constant forced adjustment of the surface formation during the chrome plating process with polishing elements. This makes it possible to prevent the enlargement of irregularities with increasing thickness of the deposit on the surface being formed, to prevent uneven distribution of the coating over the thickness, and to preserve the fine-crystalline structure of the chromium deposit (maintaining the conditions of a flat growth front of the deposit). In other words, with the CMC technology, a forced “smoothing” of the forming and growing layer of chromium deposit is performed at the microscopic level.
The conclusions of KMH development specialists from the analysis of the main chromium plating technologies existing in Russia for cylindrical long parts of the “rod” type indicate the following:
- With standard chrome plating of cylindrical parts, in order to achieve the required class of surface finish, mechanical finishing of the surface on chrome is required (using expensive equipment), which, as a rule, reduces the performance characteristics of the chrome coating (burns, scuffs, cracks);
- With CMC, a coating is formed with a roughness corresponding to the class of surface finish that is 2-3 units higher than the initial finish of the substrate. With this technology, no further mechanical processing of chromium coatings is required, dendrite formation is prevented, and accordingly the high functional properties of chromium are preserved.
The technological parameters and composition of the electrolyte of the standard technology for chrome plating of parts do not contradict the principles of the KMH technology. It should be noted that galvanic honing is not a simultaneous combination of the process of chrome plating and surface grinding, since the lapping blocks are constantly moved along the cathode surface, periodically leveling and polishing the cathode surface, without abrading part of the chromium layer as during grinding.
Hard chrome plating
The main area of application of hard chrome plating is the manufacture of tools or parts that are subject to intense mechanical stress during operation. The use of hard chrome plating significantly increases the hardness, wear resistance and service life of tools and wear parts. One of the requirements for hard chrome plating is that the steel base when applying such coatings must be hard and hardened.
Chromed tool
Here are the recommended thicknesses of hard chrome coating for various types of products: cutting tools - 5-8 microns, dies and punches of pressing equipment - 50-100 microns, molds for plastics - 10-20 microns, repair chrome plating of auto parts - up to 200 microns.
Dimensional chrome plating
Chromium deposition on the surface of the product occurs at a low rate. Thanks to this, it is possible to maintain precise layer thicknesses. The accuracy can be increased to 1 micron. The above makes it possible to bring the working parts of the measuring instrument to the required values by applying chromium without further processing. The thickness of the coating during measured chrome plating is usually 25 microns; when the tool is worn during operation to 1-2 microns of the layer thickness, the remaining chromium is removed from the surface, and the tool is re-chromed.
For measured chrome plating, an electrolyte containing chromic anhydride 150 g/l and sulfuric acid 1.5 g/l is used. and the following chrome plating modes - temperature 55-600C, cathode current density 45-100 A/dm2, voltage 12 V.
The process is carried out as follows - the parts on the suspensions are placed in an electrolyte brought to the desired temperature and heated without connecting current for 1-2 minutes, then a reverse current of the same density as the forward one is connected for 30 seconds, then the direct chrome plating process begins. The duration of chrome plating is determined experimentally.
In the working areas of the tool, after chrome plating is completed, the presence of even small chromium build-ups and uncovered areas is not allowed.
Measured chrome plating is used in the manufacture of templates, gauges and other demanding measuring instruments.
Scope of application of chrome plating
It is difficult to fully describe all areas and areas where technology is used. Chrome plating is indispensable in the furniture industry; chrome is used to process fittings and finishing elements. The technique is popular in the production of plumbing fixtures - the element is applied to the external and internal surfaces of pipes, bathtubs, sinks, and used to cover handles and faucets.
In the automotive industry, the technology is used to manufacture:
- overlays and reflectors;
- aluminum wheels;
- body elements;
- pistons;
- compression rings;
- rollers and axles.
Chrome plating is used in the production of rubber, plastics (chrome is applied to calender rolls and molds), and various measuring instruments. The material covers those elements that rub strongly against each other to increase their wear resistance.
Porous chrome plating
Porous chrome plating is used for parts that work in conjunction; the surface of the part after applying such a coating retains lubricant much better than usual. Typically, one of the rubbing parts is subjected to this type of treatment, and the antifriction properties increase significantly and the running-in of the parts improves. There are two types of porosity - channel and point. A porous chrome surface is produced by applying reverse current (anodizing), usually directly in the same bath in which the chrome plating took place. An electrolyte of standard composition is used.
The technology for producing a spot porous coating is as follows: the products are immersed in a galvanic bath, reverse current is applied and the cathode current density is maintained at 55 A/dm2 for 30 seconds, then the current density is reduced to 35 A/dm2. The duration of treatment by this method depends on the thickness of the coating (for example, with a thickness of 40 microns, the process lasts 10 minutes. Channelized porosity is obtained at a temperature increased to 650 C, the ratio of anhydride to acid content should be 115 to 1. At the end of the process, the products are removed from the bath and dried at a temperature 150-1800C for 1.5-2 hours to remove hydrogen.
Cold chrome plating
This type of chromium coating is used in cases where there is no possibility of heating the electrolyte. At room temperature, the electrolyte composition should contain 250 g/l chromic anhydride, 7-10 g/l chromium sulfate, 3 ml/l hydrofluoric acid. The cathode current density in the process is 4-5 A/dm2. The electrolyte for cold chrome plating has a high dissipative ability, but low durability. Chromium precipitation occurs slowly. It is not recommended to obtain coatings with a thickness of more than 20 microns using this method.
Combined chrome plating
With combined chrome plating, the coating has the properties of milky chrome - a coating with high anti-corrosion properties and shiny chrome - minimal porosity. Combined chrome plating is carried out in two baths, in the first one according to the milky chrome plating mode (T=700C, current density 30 A/dm2) and in the second bath, in which the products are placed without washing, according to the bright chrome plating mode (T=500C, cathode current density 40 -50 a/dm2). With combined chrome plating, the thickness of the layer of milky chrome is 15-20 microns, shiny - 35 microns.
Chrome plating of aluminum
Chromium plating of aluminum and its alloys is used to increase the wear resistance of parts and give them protective and decorative properties. The process of chrome plating of aluminum is carried out in a conventional electrolyte according to the bright chrome plating mode. The main task for obtaining a high-quality coating on aluminum is a set of preparatory operations to remove oxide films and increase the adhesion strength of the coating to the base metal. Chromium can be deposited directly on the surface of an aluminum part or on a previously applied nickel sublayer. The thickness of the coatings can vary widely - from 0.5 to 80 microns.
Do-it-yourself metal chrome plating technique
Anyone who is planning to master chrome plating of metal at home must first of all clearly understand that this chemical process involves the use of particularly toxic substances that are hazardous to health and harmful to the natural environment.
Therefore, there can be no talk of any electroplating at home. For chrome plating, it is necessary to select a non-residential premises and, if possible, equip it with at least some kind of ventilation. It is also worth taking care in advance about the disposal of the used solution and rinsing water.
All work should be performed in special clothing and using personal protective equipment used in chemical production.
VIEW Copper plates on AliExpress →
Equipment for chrome plating metal is quite easy to make yourself. In most cases, it includes:
- glass or plastic container;
- thermal insulation and sealed lid of the working container;
- heating element with thermostat;
- power supply with a power of 1 kW and a voltage of 10÷12 V;
- lead anode with terminal;
- a device for hanging and a clamp for fastening a part with a terminal;
- tanks for etching and washing, wires, stand and other minor equipment.
The layout of such a chrome plating kit depends on the size and features of the elements included in it and is done “by eye”, with additions and changes during production.
It is better to read about current modes in advance in specialized publications or communicate with knowledgeable people on specialized forums. There you can also discuss the issue of the influence of chromium on the properties of steel and other metals, since the mechanical characteristics of a chrome-plated part will change somewhat.
Preparing the surface for chrome plating
Preparing metal for chrome plating is no different from preparing for any other galvanic process. First of all, it is necessary to remove coating residues and rust from the chrome surface.
The first is done using metal brushes and sandpaper or (if possible) by abrasive blasting. You can also use mechanical methods to remove rust from metal, but it is better to use phosphoric acid.
VIEW Sprayer for chrome plating on AliExpress →
Chromium plating of aluminum and its alloys requires a special approach to pre-treatment of the surface of these metals, since they always have a stable oxide film on them. The sequence of their preparation for electroplating looks like this:
- Washing the entire metal surface in gasoline.
- Removing traces of gasoline in hot soapy water.
- Etching in a mixture of nitric and hydrofluoric acids (ratio five to one).
- Rinse in cold water.
- Placing the product in a galvanic bath.
All operations should be performed in continuous sequence, and the metal should be immersed in the electrolyte under current.
Electrolyte preparation
The main components of all electrolytes for chrome plating of metals are chromic anhydride and sulfuric acid.
Various additives are used in industrial galvanic solutions, but for the home craftsman, these two are enough for the first time. When preparing the electrolyte, sulfuric acid is first diluted in water at a rate of 1.5–2.5 g/l, and then chromic anhydride is added in an amount of 150–250 g/l. The exact proportion can only be selected experimentally, assessing the result of chrome plating of the metal surface (see also below about possible defects).
Chemical chrome plating
Chemical chromium plating is of interest primarily because, unlike the electrolytic method, it is not difficult to obtain a uniform layer of chromium using a chemical method, even on parts with complex configurations. Before chemical application of chromium, the surface of the product is treated in the same way as with galvanic application. The result is a plastic, light gray coating with high adhesive properties. The decorative properties of the chemical coating are low; a shiny surface can be obtained only after mechanical polishing of the part.
Finishing of products
Processing of products after chrome plating is carried out as follows: at the end of the process, the products are removed from the chrome plating bath and washed in cold and then in hot water. Normalization is carried out in a 3% soda solution, then washed and dried again. Parts intended to operate under heavy loads or in an aggressive environment are additionally heated for 1.5 - 2 hours at a temperature of 150-2000C to remove hydrogen, which helps to increase the strength of the chrome coating and increases the adhesion strength of chromium to the base metal. Products coated with chrome for decorative purposes are not subject to heating.
If necessary, products undergo additional mechanical processing - polishing.
Removing poor-quality coating
There are two ways to remove low-quality chrome. The first is chemical dissolution, carried out in a 50% solution of sulfuric acid. The products are placed in a container with sulfuric acid and kept until the coating is completely dissolved. The second is the anodic dissolution method, carried out in a galvanic bath. Products are immersed in a bath of 20% caustic soda solution and connected as an anode; steel sheets or parts are used as a cathode. The process takes place at a temperature of 70-800C and an anodic current density of 20-25 A/dm2 until chromium is completely dissolved. Before repeated chrome plating, the products are heated for 1.5 hours at a temperature of 150-2000C to remove hydrogen.
Necessary equipment
Tools and equipment:
- DC source with adjustable output voltage. It is permissible to process small parts when using a charger for mobile phones.
- Galvanic bath. Must be made of heat-resistant plastic or glass. The main condition is resistance to high temperatures.
- Thermometer - necessary to control the temperature during the work process.
- A heating element. The best option is a ceramic heating element. The heater must withstand prolonged exposure to acids.
For processing, it is necessary to install at least two galvanic baths so as not to constantly change the reagents in one container.
Galvanic bath (Photo: Instagram / galvaprom)
The main problems with chrome plating and methods for solving them
- Lack of coating in recessed areas of the product - occurs due to low current density in recessed areas and excess sulfuric acid in the electrolyte.
Solution: use shaped anodes, start the chrome plating process (1-2 minutes) at twice the current density - give a boost to the current, reduce the sulfuric acid content - add water or chromic anhydride to the electrolyte.
- The coating is matte or burnt (usually on protruding parts of products) - occurs due to high current density at a given temperature, passivation of anodes or insufficient heating of parts before the process.
Solution: adjust the ratio of current density and temperature, increase the distance between the anodes and cathodes, clean the anodes, control the heating of the parts before immersing them in the chrome plating bath.
- Dark stains, stripes, dots on the surface of products - insufficient concentration of sulfuric acid in the electrolyte solution
Solution: add sulfuric acid to the solution.
- The dark color of the coating means a high content of trivalent chromium, a lack of acid, and a low temperature of the electrolyte during the chrome plating process.
Solution: in addition to heating the electrolyte and adding sulfuric acid, the electrolyte should be treated with current.
- The coating peels off - poor-quality degreasing of the product surface, a sharp increase in current density with decreasing temperature.
Solution: adjust the temperature regime of chrome plating, improve the preparation of the surface of the product.
- Graininess or swelling - the presence of solid particles in the electrolyte and (or) poor preparation of the product for galvanic processing.
Solution: electrolyte filtration and quality control of parts preparation.
Basic electrolytes and modes of chrome plating of parts.
Depending on the electrolysis mode and electrolyte composition, chromium deposits with different properties can be obtained. Depending on the electrolysis conditions, three types of chromium coatings are formed:
- Gray sediments (have low physical and chemical properties and do not find practical application);
- Shiny deposits (characterized by high values of hardness and wear resistance);
- Milk sediments (least porous and most plastic).
In addition, black coatings can be obtained from special types of electrolyte.
3.1 Universal solution for chrome plating.
As a rule, “universal” chromium sulfate electrolytes are used in practice. These include: diluted, standard and concentrated electrolytes. All of the listed chromium plating electrolytes contain chromic acids H2CrO4 and H2Cr2O7, respectively, and SO42- anions in the form of sulfuric acid, as well as trivalent chromium compounds. Characteristics of electrolytes are given in Table 1.
Table 1 - Characteristics of chromium plating sulfate electrolytes.
| Electrolyte type | Concentration, g/l | Characteristic | |
| CrO3 | H2SO4 | ||
| Diluted | 150-175 | 1,5-1,75 | Current efficiency 16-18%. The dispersive power is the highest. The hardness of sediments is the highest. The composition of the electrolyte changes quickly during operation, including the ratio of components. Tendency to form rough deposits when depositing thick layers. |
| Standard | 220-250 | 2,2-2,5 | Current output 12-14%. Dispersion power is average. The working range for obtaining shiny deposits is wide. The composition of the electrolyte changes slowly, fluctuations in the ratio of components are insignificant. The hardness of the sediments is high. |
| Concentrated | 275-300 | 2,75-3,0 | Current output 8-10%. Dissipation power is low. The electrolyte is stable. The working range for obtaining shiny deposits is wide. The hardness of precipitation is the lowest. |
Table 2 shows data on the electrical conductivity of chromium plating electrolytes depending on the concentration of chromium anhydride at various temperatures.
Table 2 - Electrical conductivity of chromium plating electrolytes.
| CrO3 concentration, g/l | 100 | 200 | 300 | 400 |
| Specific electrical conductivity, S/m at a temperature of 25° C | 31,5 | 51,3 | 61,6 | 66,7 |
| Specific electrical conductivity, S/m at a temperature of 45° C | 38,9 | 63,2 | 76,3 | 81,8 |
A diluted electrolyte makes it possible to obtain high-hardness chromium deposits with relatively high current efficiency values, but at the same time rough deposits are obtained from it when thick layers are deposited (from 100-150 μm or more). In addition, ohmic voltage losses in a dilute electrolyte are the highest (electrical conductivity is low and is within 50 S/m), which directly affects the specific energy costs during chrome plating.
The standard chrome plating electrolyte is used most often in practice. At average current output values, chromium plating current modes of 3000-6000 A/m2 and temperatures of 45-70° C, thick deposits up to 300 microns are deposited from this electrolyte at lower energy costs due to higher electrical conductivity (Table 2) than from a diluted electrolyte . In addition, at temperatures of 45-55 °C, the hardness of coatings obtained from dilute electrolytes does not differ from the hardness of chromium coatings obtained from standard electrolytes.
Concentrated electrolytes are used quite rarely, as they are characterized by low current outputs during chromium deposition and low hardness of deposits (practically unsuitable for wear-resistant chromium plating).
It should be noted that in all chromium plating electrolytes, with increasing temperature, the current efficiency decreases and hydrogenation of the steel substrate increases. Hydrogenation occurs due to the fact that, simultaneously with the release of chromium, the release of hydrogen occurs on the part being coated, and most of the current is spent on this process. Hydrogenation of the steel base negatively affects its physical and mechanical properties. Hydrogen is most actively introduced into the metal in the initial period, when a continuous layer of chromium has not yet formed. When the temperature increases from 55° to 75° C, the mass of absorbed hydrogen increases 6-10 times.
The current efficiency in all chromium plating electrolytes decreases with increasing concentration of chromic anhydride. But increased concentrations of chromic anhydride make it possible to operate at higher current densities and, by increasing the current density, to intensify the chromium plating process and increase the current efficiency.
3.2 Tetrachromate chromium plating electrolyte.
This electrolyte differs from universal ones in that chromic acid is neutralized by alkali and is in the form of sodium tetrachromate. Electrolyte is recommended for use in the following cases:
- applying protective and decorative coatings without an underlayer of copper and nickel. The thickness of the chrome coating should be within 10-15 microns;
- restoration of parts by chrome plating, when high hardness and a shiny surface are not required;
- chrome plating of parts with a relatively complex configuration.
The ability to directly apply chromium from tetrachromate electrolytes to steel, brass and zinc alloys is explained by the fact that as a result of neutralization of chromic acid, the aggressiveness of the solution is noticeably reduced. Sodium tetrachromate is formed in the electrolyte by the reaction:
2 NaOH + 4CrO3 = [Na2CrO(CrO4)3] + H2O
For the process to proceed normally, it is necessary to maintain a certain ratio between the concentrations of Cr6+ and Cr3+. This ratio is regulated by the amount of reducing agent introduced.
Due to the high current density, the electrolyte becomes very hot. To avoid decomposition of tetrachromate, which is unstable at temperatures exceeding 24 ° C, the electrolyte should be cooled.
The current efficiency in this electrolyte is approximately 30%. At low current densities of 10-25 A/dm2, the deposits are soft, matte, gray, with slight internal overvoltages. With increasing current density, the current efficiency increases noticeably; with increasing temperature, the current efficiency decreases.
3.3 Self-regulating chromium plating electrolyte.
Chromium plating in electrolytes with sulfuric acid additives has a number of disadvantages. The current output of the metal is low. Usually it fluctuates between 10-12%; the composition of the electrolyte, in particular the CrO3 / H2SO4 ratio, changes during operation. The last drawback is eliminated in self-regulating electrolytes for chrome plating.
The principle of self-regulation is based on the fact that the salts of strontium sulfate and potassium silicofluoride introduced into the electrolyte have limited solubility.
These salts, being in the electrolyte in an amount exceeding their solubility, when the concentration of SO42- and SiF62- ions in the solution changes, their ratio is automatically restored.
Self-regulating electrolytes are less sensitive to temperature changes than conventional sulfuric acid electrolytes. In addition, they are relatively insensitive to contamination of the solution with iron, copper and other metals. The distribution of these electrolytes is limited by the fact that self-regulating electrolytes have a corrosive effect on some metals, including iron.
Along with the listed electrolytes, sulfate-fluoride electrolyte is used for cold chrome plating.
Quality control of chrome coatings
To determine the thickness of the chrome plating layer, standard chemical or physical control methods are used. The quality of the coating is assessed primarily visually - the coating must be even and smooth, without build-up or burnout.
This review article is intended to familiarize the customer with the chrome plating process and help in making a decision about applying one or another type of coating to their products. The administration thanks the technologist of the electroplating section S.I. Skvortsov for the knowledge provided.
You may be interested in the following articles:
|