What is chromium?
Chrome is a silvery-white metal with a bluish tint. It has high hardness and resists mechanical wear well.
Chromium is an electronegative metal; its standard electrode potential E0 (Cr0/Cr3+) is -0.74V. Under atmospheric conditions, chromium retains its color and shine for a long time, which is explained by the formation of a thin but very durable oxide film on its surface. The high tendency to passivation and the presence of this film explain the significant corrosion resistance of chromium coatings. In oxidizing environments, chromium is passivated more easily than in air and its potential shifts from the standard to the anodic region to +0.2V. The stationary electrode potential of chromium is more positive than that of iron. Therefore, chromium coatings are a cathode in relation to steel products.
Chromium is stable in a humid atmosphere, in an atmosphere of hydrogen sulfide and sulfur dioxide, in solutions of sulfuric, nitric, phosphoric and organic acids, alkalis. In solutions of hydrochloric acid and hot concentrated sulfuric acid, chromium dissolves due to the destruction of the oxide film.
Chrome plating is the process of applying a thin layer of metallic chromium to the surface of a product to impart the required characteristics. If we talk about galvanic chromium plating, metal is deposited from an electrolyte under the influence of an electric current.
Chrome coatings are widely used to give the product exceptional wear and heat resistance, as well as to reduce the coefficient of friction. Often used to give a highly decorative appearance.
| Designation | X - protective and decorative chrome plating H. TV - hard chrome plating H.mol - milky chrome plating H. m - matte chrome plating H. h - black chrome plating Chromium coating designation |
| Thickness (optimal, it is possible to deposit more) | 3-6 microns - for decorative chrome plating 15-100 microns - for hard chrome plating 24-100 microns - for milky chrome plating 15-100 microns - for matte chrome plating 1 micron (not standardized) - for black chrome plating |
| Microhardness | 7500 MPa - decorative 11000 MPa - solid 5400-6000 MPa - milk 3500 MPa - matte 2940-3430 MPa - black |
| Electrical resistivity at 18°C | 0.15*10-3 μOhm*cm |
| Permissible operating temperature | 1100oC |
| Melting temperature | 1850-1900oC. |
In compounds, chromium is most often trivalent and hexavalent. Hexavalent chromium compounds are strong oxidizing agents. Chromic anhydride, when dissolved in water, forms a mixture of chromic acids H2CrO4 and H2Cr2O7. It is from them that chrome plating of parts is most often carried out.
In a solution of chromic acid there are anions CrO42-, HCrO4-, Cr2O72-:
- at pH > 7–8 CrO42- predominate;
- at pH < 2-3 – Cr2O72-.
The electrochemical equivalent of chromium in chromic acid is 0.323 g/Ah. But, since the current efficiency of the metal in such electrolytes often does not exceed 10-12%, then in fact 0.032-0.038 g of chromium is released per 1 A*h, i.e. 30 times less than nickel, 37 times less than copper, 125 times less than silver. The only possibility of some compensation for this disadvantage is to increase the current density.
In solutions of trivalent chromium, the electrochemical equivalent of chromium is twice as large, and the current efficiency is 4-5 times greater than in chromic acid.
Numerous attempts to use electrolytes based on trivalent compounds for industrial applications, however, have not been successful, especially for the deposition of thick wear-resistant coatings.
Below, only solutions based on hexavalent chromium will be considered.
Electrochemical deposition of chromium differs significantly from other galvanic processes:
- In most electrolytes used in electroplating, the main component is the salt of the deposited metal. When chrome plating, the main component is chromic acid.
- In the chromium electrolyte, foreign anions must be present in a certain ratio: SO42-, F- or SiF62-.
- The minimum current density at which chromium precipitation begins is several hundred times higher than in other metal electrodeposition processes.
- Chromium electrodeposition is more sensitive to changes in temperature and current density compared to other galvanic processes. Changing these parameters has a significant impact not only on quantitative indicators (current efficiency, etc.), but also on the structure and properties of chromium deposits.
- Unlike other metal electrodeposition processes, the current efficiency of chromium plating decreases sharply as the temperature increases.
So, the main features of the chromium plating process are the high negative reduction potential of dichromate anions, low current yield of the metal, high operating current densities and very low dissipative ability of the electrolyte.
Electroplating mechanism.
2.1 Cathode reactions.
The mechanism of chromium electrodeposition is very complex. During chrome plating, the following processes simultaneously occur on the cathode:
- chromium precipitation;
- hydrogen release;
- reduction of hexavalent chromium to trivalent chromium;
- formation on the surface of the cathode of a thin film consisting of reduction products of chromic acid and an active anion.
It has been established that the electrolyte must contain a certain amount of active anions, without which chromium metal is not released at all.
The maximum current efficiency of chromium is achieved at a strictly defined ratio between the concentration of H2Cr2O7 and the foreign anion.
A schematic representation of cathodic polarization curves during chromium plating is shown in Figure 1. Without the addition of foreign anions, for example, sulfates, the nature of the curve is smooth (1), since hydrogen is evolved at the electrode over the entire range of current densities. When sulfuric acid is introduced into the electrolyte, the shape of the curve becomes more complicated (2).
Figure 1 - Schematic representation of cathodic polarization curves during chromium plating.
Thus, in the presence of sulfate anions, the curve consists of two branches that differ in the nature of the electrode reactions. Moreover, in the section ab
hexavalent chromium is reduced to trivalent chromium; in section
cd
, three processes occur simultaneously - reduction of hexavalent chromium to metal, reduction of hexavalent chromium to trivalent chromium, and reduction of hydrogen ions.
Activator anions change the surface state of the cathode and thus affect electrode processes. In the region of the ab
the surface becomes more active, and in the
cd
inhibition of the reduction reaction is observed. The passivity of the cathode is associated with the appearance on the surface of the electrode of a film of electrolysis products, which limits the occurrence of some reactions and promotes the occurrence of others. The abundance of hydrogen formation is explained by the low overvoltage of its evolution on chromium.
Let us consider the main processes in the chrome plating mechanism in more detail: a.
Release of hydrogen gas:
2H3O+ + 2e ↔ H2 + 2H2O , E0 = 0.0 V
b.
Acceptance of electrons by hexavalent chromium to produce di- and trivalent cations followed by precipitation of metallic chromium:
Cr2O72- + 7H2O + 6e ↔ 2Cr3+ + 14OH-, E0 = +1.33 V; Cr2O72- + 7H2O + 12e ↔ 2Cr0 + 14OH- , E0 = +0.40 V; Cr3+ + e ↔ Cr2+ , E0 = -0.41 V; Cr3+ + 3e ↔ Cr0, E0 = -0.74 V; Cr2+ + 2e ↔ Cr0, E0 = -0.91 V.
First of all, partial reduction of hexavalent chromium to the trivalent state must occur; the processes of reduction of hydrogen ions and chromium ions to the metallic state are possible together. As for divalent chromium, the presence of these ions in a free state in chromic acid, a strong oxidizing agent, is unlikely.
Researchers regarding the reduction of hexavalent chromium to metal adhere to two main views:
- The first allow for the possibility of stepwise reduction of hexavalent chromium to metal.
- The latter consider it possible to directly reduce hexavalent chromium ions to metal.
All researchers agree that during chromium plating a special film of complex composition is formed on the cathode if the coated base is prone to passivation.
V.
Synthesis of complex two-layer film.
The thin inner layer of this film is similar to the passive layers (0.0001 mm), and the outer one includes chromium in various valence states and activator ions (up to 0.025 mm). The general composition and structure of the cathode film depend on the composition and structure of the substrate being coated. For example, a cathode film does not form on copper at all, because copper easily dissolves in the electrolyte, and on iron and nickel it is especially pronounced due to the enhanced passivation of these metals by chromic acid. The cathode film is colloidal in nature; its composition can change with changes in current density and electrolyte temperature. In general, it can contain: up to 67% hexavalent chromium, up to 23% trivalent chromium, up to 12% sulfate ions. The film thickness increases with increasing foreign anion concentration. The structure of the coating depends on the properties of the film, and its properties depend on the structure. It is at this stage that mechanical and chemical differences between different chromium deposits are formed. For example, the structure of the cathode film at low current densities and high temperatures creates favorable conditions for the production of milky chromium.
The mechanism of the influence of foreign anions on the chromium plating process has not been clarified in detail. There are two hypotheses explaining their influence:
- According to the first of them, anions are activators that cause activation of the cathode surface. In the absence of these ions, the surface is covered with Cr3+ compounds, which prevents the complete reduction of H2Cr2O7 and the deposition of chromium metal on the cathode.
- According to the second hypothesis, foreign anions form reaction complexes with H2Cr2O7 that have a greater ability to reduce the latter.
Based on a number of studies using the radioactive isotope Cr3+, it was concluded that the electrodeposition of chromium occurs from its hexavalent ions. It was established that if Cr3+ was present in the electrolyte in the form of its trivalent compounds, the precipitate was non-radioactive. It has already been noted that it is not possible to obtain high-quality precipitates from solutions of trivalent chromium salts. It is possible that in an electric field near the cathode, anions such as Cr2O72- and Cr2O42- are deformed, turning their positive core towards the cathode. At a sufficiently high potential, Cr(VI) can be torn out from the anion with a transition to the metal lattice. The rate of this electrochemical reaction is determined by the overvoltage and the concentration of CrO42- at the cathode surface. Considering the structure of Cr2O72-, it is not difficult to notice that the deformation of their ions and the removal of Cr(VI) should be difficult. Therefore, it is quite possible that the first process occurring at low current densities is the reduction of Cr2O72- anions to Cr3+ ions with simultaneous alkalization of solutions. Upon reaching the limiting current for the Cr2O72- discharge, hydrogen evolution begins, in which case further alkalization of the solution takes place. As a result of this, the pH of the catholyte reaches 5-7, and then CrO42- ions begin to predominate at the cathode, which, when deformed, can be restored both partially and completely:
CrO42- + 3e + 4 H2O = Cr(OH)3 + 5OH- CrO42- + 6e + 4 H2O = Cr0 + 8OH-
The role of SO42- is probably limited to the dissolution of chromium hydroxide or other colloidal compounds created by trivalent chromium, because they can form a soluble complex Cr4(SO4)4(H2O)2+. SO42- ions do not participate in the cathodic process, because Direct measurements show that their concentration in the near-cathode space does not change during the electrolysis process. A large number of SO42- ions worsens deposition, since in this case, apparently, CrO42- can be displaced by them from the surface, and the current efficiency decreases.
2.2 Anodic reactions on insoluble anodes.
When chrome plating parts, insoluble anodes are used. This is explained by the fact that chromium dissolves at the anode with a higher current output than is deposited at the cathode, and goes into solution in the form of ions of different valences.
Anodic reactions during chrome plating with the participation of insoluble lead anodes are as follows:
Pb + SO42- - 2e = PbSO4, E0 = +0.356 B; Pb + 4H2O - 4e = PbO2 + 4H+, E0 = +0.665 B; H2O - 2e = ½O2 + 2H+, E0 = +1.228 V; PbSO4 + 2H2O - 2e = PbO2 + SO42- + 4H+, E0 = +1.185 B; 2Cr3+ - 6e + 7H2O = Cr2O72- + 14H+, E0 = +1.300 V.
During the electrolysis process, the anodes are coated with a layer of lead (IV) oxide PbO2, which catalyzes the Cr3+ → Cr6+ process and protects the anodes from destruction. At a certain ratio of the anodic and cathodic current densities, it is possible to establish an equilibrium in which the same amount of trivalent chromium will be oxidized at the anode as enters the electrolyte from the cathode zone.
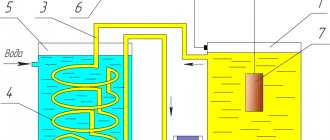
Necessary equipment
Tools and equipment:
- DC source with adjustable output voltage. It is permissible to process small parts when using a charger for mobile phones.
- Galvanic bath. Must be made of heat-resistant plastic or glass. The main condition is resistance to high temperatures.
- Thermometer - necessary to control the temperature during the work process.
- A heating element. The best option is a ceramic heating element. The heater must withstand prolonged exposure to acids.
For processing, it is necessary to install at least two galvanic baths so as not to constantly change the reagents in one container.
Galvanic bath (Photo: Instagram / galvaprom)
Basic electrolytes and modes of chrome plating of parts.
Depending on the electrolysis mode and electrolyte composition, chromium deposits with different properties can be obtained. Depending on the electrolysis conditions, three types of chromium coatings are formed:
- Gray sediments (have low physical and chemical properties and do not find practical application);
- Shiny deposits (characterized by high values of hardness and wear resistance);
- Milk sediments (least porous and most plastic).
In addition, black coatings can be obtained from special types of electrolyte.
3.1 Universal solution for chrome plating.
As a rule, “universal” chromium sulfate electrolytes are used in practice. These include: diluted, standard and concentrated electrolytes. All of the listed chromium plating electrolytes contain chromic acids H2CrO4 and H2Cr2O7, respectively, and SO42- anions in the form of sulfuric acid, as well as trivalent chromium compounds. Characteristics of electrolytes are given in Table 1.
Table 1 - Characteristics of chromium plating sulfate electrolytes.
| Electrolyte type | Concentration, g/l | Characteristic | |
| CrO3 | H2SO4 | ||
| Diluted | 150-175 | 1,5-1,75 | Current efficiency 16-18%. The dispersive power is the highest. The hardness of sediments is the highest. The composition of the electrolyte changes quickly during operation, including the ratio of components. Tendency to form rough deposits when depositing thick layers. |
| Standard | 220-250 | 2,2-2,5 | Current output 12-14%. Dispersion power is average. The working range for obtaining shiny deposits is wide. The composition of the electrolyte changes slowly, fluctuations in the ratio of components are insignificant. The hardness of the sediments is high. |
| Concentrated | 275-300 | 2,75-3,0 | Current output 8-10%. Dissipation power is low. The electrolyte is stable. The working range for obtaining shiny deposits is wide. The hardness of precipitation is the lowest. |
Table 2 shows data on the electrical conductivity of chromium plating electrolytes depending on the concentration of chromium anhydride at various temperatures.
Table 2 - Electrical conductivity of chromium plating electrolytes.
| CrO3 concentration, g/l | 100 | 200 | 300 | 400 |
| Specific electrical conductivity, S/m at a temperature of 25° C | 31,5 | 51,3 | 61,6 | 66,7 |
| Specific electrical conductivity, S/m at a temperature of 45° C | 38,9 | 63,2 | 76,3 | 81,8 |
A diluted electrolyte makes it possible to obtain high-hardness chromium deposits with relatively high current efficiency values, but at the same time rough deposits are obtained from it when thick layers are deposited (from 100-150 μm or more). In addition, ohmic voltage losses in a dilute electrolyte are the highest (electrical conductivity is low and is within 50 S/m), which directly affects the specific energy costs during chrome plating.
The standard chrome plating electrolyte is used most often in practice. At average current output values, chromium plating current modes of 3000-6000 A/m2 and temperatures of 45-70° C, thick deposits up to 300 microns are deposited from this electrolyte at lower energy costs due to higher electrical conductivity (Table 2) than from a diluted electrolyte . In addition, at temperatures of 45-55 °C, the hardness of coatings obtained from dilute electrolytes does not differ from the hardness of chromium coatings obtained from standard electrolytes.
Concentrated electrolytes are used quite rarely, as they are characterized by low current outputs during chromium deposition and low hardness of deposits (practically unsuitable for wear-resistant chromium plating).
It should be noted that in all chromium plating electrolytes, with increasing temperature, the current efficiency decreases and hydrogenation of the steel substrate increases. Hydrogenation occurs due to the fact that, simultaneously with the release of chromium, the release of hydrogen occurs on the part being coated, and most of the current is spent on this process. Hydrogenation of the steel base negatively affects its physical and mechanical properties. Hydrogen is most actively introduced into the metal in the initial period, when a continuous layer of chromium has not yet formed. When the temperature increases from 55° to 75° C, the mass of absorbed hydrogen increases 6-10 times.
The current efficiency in all chromium plating electrolytes decreases with increasing concentration of chromic anhydride. But increased concentrations of chromic anhydride make it possible to operate at higher current densities and, by increasing the current density, to intensify the chromium plating process and increase the current efficiency.
3.2 Tetrachromate chromium plating electrolyte.
This electrolyte differs from universal ones in that chromic acid is neutralized by alkali and is in the form of sodium tetrachromate. Electrolyte is recommended for use in the following cases:
- applying protective and decorative coatings without an underlayer of copper and nickel. The thickness of the chrome coating should be within 10-15 microns;
- restoration of parts by chrome plating, when high hardness and a shiny surface are not required;
- chrome plating of parts with a relatively complex configuration.
The ability to directly apply chromium from tetrachromate electrolytes to steel, brass and zinc alloys is explained by the fact that as a result of neutralization of chromic acid, the aggressiveness of the solution is noticeably reduced. Sodium tetrachromate is formed in the electrolyte by the reaction:
2 NaOH + 4CrO3 = [Na2CrO(CrO4)3] + H2O
For the process to proceed normally, it is necessary to maintain a certain ratio between the concentrations of Cr6+ and Cr3+. This ratio is regulated by the amount of reducing agent introduced.
Due to the high current density, the electrolyte becomes very hot. To avoid decomposition of tetrachromate, which is unstable at temperatures exceeding 24 ° C, the electrolyte should be cooled.
The current efficiency in this electrolyte is approximately 30%. At low current densities of 10-25 A/dm2, the deposits are soft, matte, gray, with slight internal overvoltages. With increasing current density, the current efficiency increases noticeably; with increasing temperature, the current efficiency decreases.
3.3 Self-regulating chromium plating electrolyte.
Chromium plating in electrolytes with sulfuric acid additives has a number of disadvantages. The current output of the metal is low. Usually it fluctuates between 10-12%; the composition of the electrolyte, in particular the CrO3 / H2SO4 ratio, changes during operation. The last drawback is eliminated in self-regulating electrolytes for chrome plating.
The principle of self-regulation is based on the fact that the salts of strontium sulfate and potassium silicofluoride introduced into the electrolyte have limited solubility.
These salts, being in the electrolyte in an amount exceeding their solubility, when the concentration of SO42- and SiF62- ions in the solution changes, their ratio is automatically restored.
Self-regulating electrolytes are less sensitive to temperature changes than conventional sulfuric acid electrolytes. In addition, they are relatively insensitive to contamination of the solution with iron, copper and other metals. The distribution of these electrolytes is limited by the fact that self-regulating electrolytes have a corrosive effect on some metals, including iron.
Along with the listed electrolytes, sulfate-fluoride electrolyte is used for cold chrome plating.
Structure and composition of chrome coatings.
By changing the electrolysis mode, it is possible to obtain different types of chromium deposits, differing in their properties and, therefore, in their scope of application. The greatest technical and economic effect is achieved when using wear-resistant and corrosion-resistant chromium plating. It is important to note that hardness does not always correlate with wear resistance.
The diagrams in Figure 2 clearly demonstrate the areas of obtaining hard and wear-resistant coatings under standard chromium plating modes in “universal” sulfate electrolytes.
Figure 2 - Diagrams of conditions for obtaining hard (T) and wear-resistant (I) chromium coatings under standard chromium plating modes in “universal” sulfate electrolytes: in a diluted electrolyte (a) and a standard electrolyte (b).
The wear resistance of coatings obtained from a “universal” standard electrolyte increases with increasing temperature and, having passed through a maximum at 55-65 ° C, decreases to a minimum at 75 ° C. For deposits obtained from a dilute electrolyte, the maximum wear resistance shifts to the region of higher temperatures The ductility of electrolytic chromium also significantly depends on the chromium plating mode. Brittle chromium deposits (shiny and matte) are obtained at low electrolyte temperatures and high current densities, while more plastic coatings are obtained at high temperatures and low current densities (milky deposits).
Electrolytic chromium deposits have an extremely fine crystalline structure (Figure 3).
Figure 3 - Microimage (from left to right) of hard, thin shiny and milky crack-free chrome, x100.
The smallest sizes (0.001-0.01 microns) are crystals of shiny chromium. The sizes of matte and milky chromium crystals are 0.1-10 microns.
Below are photographs of microsections of a cross section of a chromium layer at 1000x magnification (Figure 4).
a b
Figure 4 - Microphotographs of transverse sections of matte (a) and shiny chrome (b).
Electrolytically deposited chromium contains oxygen, hydrogen and trace amounts of nitrogen. The mass fraction of oxygen is 0.2-0.5 mass fractions, hydrogen - 0.03-0.07 mass fractions. The volume of gases included in the deposit depends on temperature and current density: with increasing temperature and decreasing current density, the volume of gases in the deposit decreases slightly.
Two main structural modifications of electrolytically deposited chromium are known:
- α-Cr with a cubic body-centered crystal lattice and a density of 7.1 g/cm3
- β-Cr with a close-packed hexagonal crystal lattice and a density of 6.08 g/cm3.
At high current densities and elevated electrolyte temperatures, a predominantly cubic α-Cr structure is formed; at low current densities and room temperature, a predominantly hexagonal β-Cr structure is formed. This structure is stable only at temperatures below 25° C, and at higher temperatures it transforms into a stable α-Cr modification.
Chromium deposits are characterized by high internal stresses, the cause of which is the spontaneous transition of unstable β-Cr into the stable modification α-Cr, which has a higher density, as a result of which a network of microcracks is formed in the chromium deposits. As the electrolyte temperature increases, internal stresses and the number of cracks decrease.
All chromium coatings are characterized by high hardness, but the hardness of cubic chromium is significantly higher than the hardness of hexagonal chromium. Hardness is determined by the electrolysis mode, as mentioned above.
Finishing of products
Processing of products after chrome plating is carried out as follows: at the end of the process, the products are removed from the chrome plating bath and washed in cold and then in hot water. Normalization is carried out in a 3% soda solution, then washed and dried again. Parts intended to operate under heavy loads or in an aggressive environment are additionally heated for 1.5 - 2 hours at a temperature of 150-2000C to remove hydrogen, which helps to increase the strength of the chrome coating and increases the adhesion strength of chromium to the base metal. Products coated with chrome for decorative purposes are not subject to heating.
If necessary, products undergo additional mechanical processing - polishing.
Cathodic mechanical chrome plating (galvanic honing).
An analysis of modern literary sources covering the issues of intensification of chrome plating processes, as well as modern Russian chrome plating technologies, has shown that applying shiny chrome coatings to cylindrical parts or rod-type parts made from a standard sulfate electrolyte at current densities of 3000-6000 A/m2 and electrolyte temperatures of 45 -70 °C allows the technology of cathodic mechanical chromium plating (CMC) or galvanic honing. This technology was developed by specialists from the Federal State Unitary Enterprise “TsNIIM” (St. Petersburg).
KMH technology involves chrome plating of cylindrical parts with a simultaneous mechanical (abrasive) effect on the cathode surface, that is, combining the chrome plating process with honing or lapping the surface with special polishing elements. According to developers' estimates, the wear resistance of chrome coatings obtained using the CMC technology, compared with coatings obtained by standard chrome plating of cylindrical parts, increases by 2-4 times [4]. In addition, the use of cathodic mechanical chromium plating makes it possible to obtain thick-layer chrome coatings (thickness over 100 microns) with a roughness corresponding to high classes of surface finish (not lower than class 9) without intermediate mechanical treatment.
The essence of the galvanic honing process is a constant forced adjustment of the surface formation during the chrome plating process with polishing elements. This makes it possible to prevent the enlargement of irregularities with increasing thickness of the deposit on the surface being formed, to prevent uneven distribution of the coating over the thickness, and to preserve the fine-crystalline structure of the chromium deposit (maintaining the conditions of a flat growth front of the deposit). In other words, with the CMC technology, a forced “smoothing” of the forming and growing layer of chromium deposit is performed at the microscopic level.
The conclusions of KMH development specialists from the analysis of the main chromium plating technologies existing in Russia for cylindrical long parts of the “rod” type indicate the following:
- With standard chrome plating of cylindrical parts, in order to achieve the required class of surface finish, mechanical finishing of the surface on chrome is required (using expensive equipment), which, as a rule, reduces the performance characteristics of the chrome coating (burns, scuffs, cracks);
- With CMC, a coating is formed with a roughness corresponding to the class of surface finish that is 2-3 units higher than the initial finish of the substrate. With this technology, no further mechanical processing of chromium coatings is required, dendrite formation is prevented, and accordingly the high functional properties of chromium are preserved.
The technological parameters and composition of the electrolyte of the standard technology for chrome plating of parts do not contradict the principles of the KMH technology. It should be noted that galvanic honing is not a simultaneous combination of the process of chrome plating and surface grinding, since the lapping blocks are constantly moved along the cathode surface, periodically leveling and polishing the cathode surface, without abrading part of the chromium layer as during grinding.
Surface preparation
This important stage should be given special attention. After all, you can get a high-quality coating only on perfectly cleaned and grease-free surfaces. Therefore, before chrome-plating metal, wood or plastic at home, they must be thoroughly cleaned.
The parts being processed should be free of dust, dirt, and the slightest traces of rust. Old coating and paint should also be completely removed.
After cleaning, uneven surfaces are thoroughly sanded. There should be no chips or scratches left. The next stage is polishing with sandpaper and special pastes.
Then the part to be chrome-plated is degreased in a solution heated almost to boiling (90°C). Products made of non-ferrous metals can be treated with a mixture of soap without cosmetic additives and sodium phosphate. Steel or cast iron is immersed in a solution of liquid glass, caustic soda, sodium carbonate and sodium phosphate. Sulfuric acid is used to remove the oxide film.