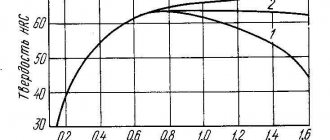
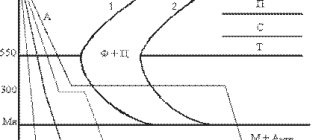
You may have heard these terms more than once when talking about forged knives, and steels in general. It's time to figure out what they mean.
Hardening, in its essence, is heating the finished product to a certain temperature, followed by cooling at a certain speed, and tempering is additional heating following hardening to lower temperatures with a different cooling mode; exactly which one depends on the grade of steel. The speed is regulated by the so-called. “quenching medium” - a liquid in which the blade is cooled at a certain speed: machine oil, saline solutions, air flow, etc. For example, oil cools at a rate approximately 6 times slower than circulating water.
To get to specific numbers, you need to understand why these two processes are needed at all.
General information about steel hardening technology
The main goals achieved by the hardening + tempering complex:
- increased hardness;
- increasing strength characteristics;
- reduction of ductility to an acceptable value;
- the possibility of using hollow products instead of solid ones, which allows reducing the weight of the metal product and the metal intensity of the production process.
Main stages of hardening:
- heating to temperatures at which the structural state of the metal changes;
- shutter speed set in the technological map;
- cooling at a rate that ensures the formation of a given crystal structure.
After hardening, tempering is carried out, which consists of heating the metal to temperatures below the line of phase transformations, with a further slow decrease in temperature. The result of heat treatment is influenced by:
- heating temperature;
- rate of temperature rise;
- holding period at quenching temperatures;
- cooling medium and rate of temperature decrease.
The key parameter is the heating temperature, on which the restructuring and formation of a new structural lattice depends. Based on the depth of action, hardening is divided into volumetric and surface. In mechanical engineering, volumetric hardening is usually used, after which the hardness of the surface and core differs slightly. Surface heat treatment is in demand for parts for which high surface hardness and a viscous core are important.
What steels are hardened?
Not all steel grades can be hardened. Grades with a carbon content below 0.4% practically do not change hardness at quenching temperatures, so this method is not used for them. Hardening technology is most often used for tool steels.
Table of correct quenching and tempering conditions for certain types of tool steels
| steel grade | Steel hardening temperature | Cooling medium after quenching heating | Holiday temperature | Cooling environment after tempering |
| U7 | 800°C | water | 170°C | water, oil |
| U7A | 800°C | water | 170°C | water, oil |
| U8, U8A | 800°C | water | 170°C | water, oil |
| U10, U10A | 790°C | water | 180°C | water, oil |
| U11, U12 | 780°C | water | 180°C | water, oil |
| P9 | 1250°C | oil | 580°C | air in the oven |
| P18 | 1250°C | oil | 580°C | air in the oven |
| ШХ6 | 810°C | oil | 200°C | air |
| ШХ15 | 845°C | oil | 400°C | air |
| 9ХС | 860°C | oil | 170°C | air |
What kind of steel can be hardened
Heat treatment of metals is one of the main ways to improve their mechanical and physical-chemical characteristics: hardness, strength and others.
One type of heat treatment is hardening. It has been successfully used by man in a handicraft way since ancient times. In the Middle Ages, this method of heat treatment was used to improve the strength and hardness of metal household items: axes, sickles, saws, knives, as well as military weapons in the form of spears, sabers and others.
And now they use this method of improving the characteristics of metal, not only on an industrial scale, but also at home, mainly for hardening metal household items.
Types of hardening - with and without polymorphic transformation
Hardening of steels proceeds with a polymorphic transformation, non-ferrous metals and alloys - without them.
Hardening of steels with polymorphic transformation
In carbon steels, when temperatures rise above a certain level, a series of phase transformations occur, causing changes in the crystal lattice. At critical temperatures, the value of which depends on the percentage of carbon, the decomposition of iron carbide occurs and the formation of a solution of carbon in iron, called austenite. With slow cooling, austenite gradually disintegrates, and the crystal lattice acquires its original state. If carbon steels are cooled at a high speed, then, depending on the quenching mode, various phase states are formed in them, the strongest of which is martensite.
To obtain a martensitic structure, hypoeutectoid steels (up to 0.8% C) are heated to temperatures above the Ac3 point by 30-50°C, for hypereutectoid steels - 30-50° above Ac1. Using this technology, metal-cutting tools are hardened and products are strengthened, which are subject to friction during operation: gears, shafts, races, bushings. When heated to lower temperatures, the structure of hypoeutectoid steels, along with martensite, retains softer ferrite, which reduces the hardness of the metal and worsens its mechanical characteristics after tempering. Such hardening of steel is called incomplete and in most cases is a defect. But it can be used in some cases to avoid cracks.
Hardening without polymorphic transformation
Hardening without polymorphic transformation occurs in non-ferrous metals and alloys that have limited solubility of secondary phases at ordinary temperatures, in which polymorphic transformations do not occur at high temperatures. When temperatures increase above the solidus line (this is the line below which only the solid phase is located), the secondary phases completely dissolve. During rapid cooling, secondary phases do not separate out, since this requires a certain time. After such heat treatment, the non-ferrous alloy is thermodynamically unstable, so over time it begins to decompose with the gradual release of a secondary phase. This decomposition process, which occurs under natural conditions, is called natural aging, and when heated, it is called artificial aging. As a result of aging, an equilibrium structure is obtained. The characteristics of the material depend on the selected process mode.
Hardening of non-ferrous metals and alloys, unlike carbon steels, often does not lead to an increase in strength. Copper-based alloys, for example, often become more ductile after such maintenance. For such materials, tempering is usually used, which relieves stress after casting, rolling, stamping, forging or pressing.
Characteristics of steel: hardenability and hardenability
The important characteristics of steel - hardenability and hardenability - should not be confused.
Hardenability
This characteristic indicates the ability of steel to gain hardness after hardening. There are types of steel that are difficult to harden and after the heat treatment process the steel becomes insufficiently hard. They say about such material that it “did not accept hardening.”
The hardness of martensite is related to the degree of distortion of its crystal lattice. A lower carbon content in martensite contributes to less distortion in the crystal lattice, which means the hardness of the steel will be lower. If the steel contains less than 0.3% carbon, then the hardenability of such an alloy is low, and usually such alloys are not hardened.
Hardenability
This characteristic can indicate how deeply the steel has been hardened. When hardening, the surface of a steel part cools faster than the core . This occurs because the surface is in direct contact with the cooling fluid, which removes heat. And the central part of the steel part gives off its heat through the thickness of the metal and the surface, where it is absorbed by the coolant.
Hardenability is affected by the critical hardening rate - the lower it (speed), the deeper the steel is hardened. For example, coarse-grained steel, which has a low critical hardening rate, is annealed deeper than fine-grained steel, which has a high critical hardening rate.
The depth of hardenability depends on the initial structure of the alloy being hardened, the heating temperature and the quenching medium. The hardenability of steel is determined by fracture, microstructure and hardness.
Steel hardening methods
The hardening method is chosen depending on the chemical composition of the steel and the planned properties.
Hardening with cooling in one environment
The rate of cooling of steel after hardening depends on the environment in which it is carried out. The highest speed is provided by cooling in water. This method is used for medium-carbon low-alloy steels and some grades of corrosion-resistant steels. When the carbon content is more than 0.5% C and high alloying, water is not used as a cooling medium, since such alloys become cracked or completely destroyed.
Intermittent quenching in two cooling media
Step hardening is used for parts made of complex alloy steels. Large parts, after heating, are dipped in water for several minutes, and then cooled in oil to +320...300°C, after which they are left in the air. When cooled in oil to room temperature, the hardness of the product decreases significantly.
Isothermal maintenance
Hardening of high-carbon grades is a complex process consisting of normalization followed by heating to the hardening temperature. The heated parts are lowered into a bath of saltpeter, heated to temperatures of +320...+350°C, and kept.
Svetlaya TO
This heat treatment is used for high-alloy steels and consists of heating them in an environment of inert gases or in a vacuum, which ensures a light-colored metal surface. Light hardening is used in mass production of standard products.
Heat treatment with self-tempering
At a high cooling rate, heat remains inside the part, which, when gradually released, relieves stress on the internal structure. This process can only be entrusted to specialists who can accurately calculate the time the product is in the cooling environment.
Jet
Cooling is carried out with an intense stream of water. This process is used when it is necessary to harden individual parts of products.
How to harden metal at home
Using basic knowledge, you can harden steel at home. Heating of metal is usually carried out using a fire, electric muffle furnaces or gas burners.
Hardening an ax at the stake and in the oven
If you need to give additional strength to household tools, for example, to make an ax more durable, then the easiest way to harden it can be done at home.
During manufacture, axes are stamped with a mark by which you can identify the grade of steel. We will look at the hardening process using U7 tool steel as an example.
The technology must be performed in compliance with the following rules:
1. Annealing . Before processing, dull the sharp edge of the blade and place the ax in a burning brick oven to heat. The heat treatment procedure must be carefully monitored to prevent overheating (permissible heating is 720-780°C). More advanced craftsmen recognize the temperature by the color of the heat.
And beginners can find out the temperature using a magnet. If the magnet stops sticking to the metal, it means the ax has heated up above 768°C (red-burgundy color) and it’s time to cool down.
Use a poker to move the hot ax to the oven door, remove the heat deeper, close the door and valve, leave the heated metal in the oven for 10 hours. Let the ax gradually cool down with the stove.
2. Hardening of steel . Heat the ax over a fire, potbelly stove or stove until dark red - temperature 800-830°C (the magnet has stopped magnetizing, wait another 2-3 minutes).
Quenching is performed in heated water (30°C) and oil. Lower the ax blade into the water 3-4 cm, moving it vigorously.
Next, place the ax in a container with oil; if the oil catches fire, cover the container with a thick cloth. Keep in oil until completely cool.
3. Release of the ax blade . Tempering reduces the brittleness of steel and relieves internal stress. Sand the metal with sandpaper to better distinguish the colors of the paint.
Place the ax in the oven for 1 hour at a temperature of 270-320°C. After standing, remove and cool in air.
Video: heat treatment of an ax at home, three stages: annealing, hardening, tempering.
Hardening the knife
It is advisable to use furnaces to harden metals yourself. For household items in the form of knives, axes, drills and others, small muffle furnaces are the most suitable. In them you can achieve a hardening temperature much higher than on a fire and it is easier to achieve uniform heating of the metal.
You can make such a stove yourself. You can find many simple design options on the Internet. In such ovens, a metal product can be heated to 700-900°C.
Let's look at how to harden a stainless steel knife at home using an electric muffle furnace. For cooling, instead of water or oil, melted sealing wax is used (can be obtained from a military unit).
The sequence of the hardening process is as follows:
- the knife (without a handle, if it is wooden) is placed in a cold oven;
- turning on the closed oven, heat it together with the knife until the blade turns bright red (800-900°C);
- Using a hot knife blade, cut the sealing wax up to 10 times, plunging 1.5 cm into it;
- the procedure is repeated up to 5 times, heating the knife blade and cooling in wax;
- Remains of sealing wax are removed with turpentine using a dampened cloth.
It is better to do the procedure in the fresh air; sealing wax smells terrible when melted. Also, the knife blade can be heated over an open fire.
Video: other ways to harden a knife at home.
Equipment for hardening
The equipment is divided into two main groups - heating units and cooling baths. At modern enterprises, the following are used to obtain quenching temperatures:
- thermal muffle furnaces;
- induction heating equipment;
- installations for heating in melts;
- laser heating devices;
- gas plasma devices.
The first three types of installations are in demand for volumetric hardening, the last three - for the surface process.
Quenching equipment is steel containers, graphite crucibles, furnaces that contain molten metals or salts. Quenching baths for liquid media are equipped with heating and cooling systems. Their design may include special mixers for mixing liquid media and eliminating the steam jacket.
Quenching media [edit | edit code]
When quenching, to supercool austenite to the martensitic transformation temperature, rapid cooling is required, but not over the entire temperature range, but only within 650–400 °C, that is, in the temperature range in which austenite is least stable and most quickly transforms into ferrite. cementite mixture. Above 650 °C, the rate of austenite transformation is low, and therefore the mixture during quenching can be cooled in this temperature range slowly, but, of course, not so much that ferrite precipitation or the transformation of austenite into pearlite begins.
The mechanism of action of quenching media (water, oil, water-polymer quenching media, as well as cooling of parts in salt solutions) is as follows. At the moment the product is immersed in the quenching medium, a film of superheated steam forms around it; cooling occurs through the layer of this steam jacket, that is, relatively slowly. When the surface temperature reaches a certain value (determined by the composition of the quenching liquid), at which the steam jacket ruptures, the liquid begins to boil on the surface of the part, and cooling occurs quickly.
Hardening process technology
Heating and holding
The heating temperature of steel during hardening depends on its chemical composition. In general, there is a pattern - the lower the percentage of carbon, the higher the heating temperature should be. Lowering the heating temperature leads to the fact that the desired structure does not have time to form. Consequences of overheating:
- decarbonization;
- surface oxidation;
- increase in internal tension;
- change in structural components.
Products of complex shapes are preheated. To do this, they are dipped two or three times in salt baths for several minutes or kept for a short time in ovens heated to temperatures of +400...500°C. The holding period is determined by the dimensions of the product and their quantity in the oven. All parts of the product must be heated evenly.
Table of hardening temperatures for various steel grades
| Brand | Temperature, °C | Brand | Temperature, °C |
| 15G | 800 | 50G2 | 805 |
| 65G | 815 | 40ХГ | 870 |
| 15X, 20X | 800 | 3Х13 | 1050 |
| 30Х, 35Х | 850 | 35ХГС | 870 |
| 40Х, 45Х | 840 | 30ХГСА | 900 |
| 50X | 830 |
The heating temperature is measured using pyrometers - contact and non-contact, infrared devices.
Cooling
For cooling, water is used - pure or with salts dissolved in it, alkaline solutions. For alloy steels, blowing or cooling in mineral oils is used. In isothermal and stepwise processes, molten salts, alkalis and metals are used for cooling. Such environments can alternate with each other.
Vacation
Depending on the required temperature, tempering is carried out in oil, alkaline or saltpeter baths, furnaces with forced circulation of air flows, and hot sand.
Low tempering, carried out at +150...+200°C, serves to eliminate internal stresses, slightly increase ductility and toughness without significantly deteriorating hardness. Low tempering is in demand for measuring and metalworking tools, and other parts that must combine hardness and wear resistance.
For high-speed steels, tempering is carried out at temperatures of +550...580°C. This procedure is called secondary hardening because it leads to an additional increase in hardness.
Possible defects after hardening
Heating, holding, cooling and tempering of steel are carried out in accordance with technological maps developed by specialists. Violation of the developed and approved technical process and/or heterogeneity of the workpiece structure can cause various defects. Among them:
- Uneven heating and/or cooling. They lead to deformations and the formation of cracks, non-uniform composition and non-uniform mechanical characteristics.
- Burnout. Occurs due to the penetration of oxygen molecules into the metal surface. As a result, oxides are formed that change the performance characteristics of the surface layer. This defect occurs due to the burning of carbon from the steel caused by excess oxygen in the furnace.
- Water entering the oil cooling bath. This violation of the technical process leads to the appearance of cracks in the product.
All of the above defects are irreparable.