Thermal (or heat) treatment
is a set of operations of heating, holding and cooling hard metal alloys in order to obtain specified properties by changing the internal structure and structure. Heat treatment is used either as an intermediate operation to improve workability by pressure or cutting, or as the final operation of a technological process, ensuring a given level of product properties.
The total duration of heating of the metal during heat treatment is the sum of the time of its own heating to a given temperature and the holding time at this temperature. The heating time depends on the type of oven, the size of the products, and their placement in the oven; the holding time depends on the rate of phase transformations.
Heating can be accompanied by interaction of the metal surface with a gaseous environment and lead to decarburization of the surface layer and the formation of scale. Decarburization causes the surface of products to become less durable and lose hardness.
When heating and cooling steel, phase transformations occur, which are characterized by temperature critical points. It is customary to designate the critical points of steel with the letter A. Critical points A1 lie on the PSK line (727 °C) of the iron-carbon diagram and correspond to the transformation of pearlite into austenite. Critical points A2 are located on the MO line (768 °C), which characterizes the magnetic transformation of ferrite. A3 corresponds to lines GS and SE, where the transformation of ferrite and cementite into austenite upon heating is completed, respectively.
To designate critical points during heating and cooling, additional indices are introduced: the letter “c” in the case of heating and “r” in the case of cooling, for example Ac1, Ac3, Ar1, Ar3.
Types of heat treatment [edit | edit code ]
Among the main types of heat treatment, it should be noted:
- Annealing
- Annealing of the 1st kind (homogenization, recrystallization, stress relief). The goal is to obtain an equilibrium structure. Such annealing is not associated with transformations in the solid state (if they occur, then this is a side effect).
- Second-order annealing is associated with transformations in the solid state. The 2nd type of annealing includes: complete annealing, partial annealing, normalization, isothermal annealing, patenting, spheroidizing annealing.
- Quenching is carried out with an increased cooling rate in order to obtain nonequilibrium structures. The critical cooling rate required for quenching depends on the chemical composition of the alloy. Hardening can be accompanied by a polymorphic transformation, in which a new nonequilibrium phase is formed from the initial high-temperature phase (for example, the transformation of austenite into martensite during steel quenching). There is also hardening without a polymorphic transformation, during which a high-temperature metastable phase is fixed (for example, when hardening beryllium bronze, the alpha phase supersaturated with beryllium is fixed).
- Tempering is necessary to relieve internal stresses, as well as to give the material the required set of mechanical and operational properties. In most cases, the material becomes more ductile with a slight decrease in strength.
- Normalization. The product is heated to the austenitic state (30...50 degrees above AC3) and cooled in still air
- Dispersion hardening (aging). After hardening (without polymorphic transformation), heating is carried out at a lower temperature in order to release particles of the strengthening phase. Sometimes stepwise aging is carried out at several temperatures in order to isolate several types of strengthening particles.
- Cryogenic treatment is a hardening heat treatment of metal products at cryogenic, ultra-low temperatures (below minus 153°C).
- increasing the wear resistance of tools, parts and mechanisms
- reducing the number of defects
- reducing costs for repairs and replacement of technological equipment and tools.
Previously, different terminology was used to refer to this process - “cold treatment”, “heat treatment of steel at temperatures below zero”, but they did not accurately reflect the essence of the cryogenic processing process. The essence of cryogenic processing is as follows: parts and mechanisms are placed in a cryogenic processor, where they are slowly cooled and then maintained at a temperature of minus 196˚C for a certain time. Then the processed products gradually return to room temperature. During this process, structural changes occur in the metal. They significantly increase wear resistance, cyclic strength, corrosion and erosion resistance. This technology makes it possible to increase the service life of tools, parts and mechanisms by up to 300% by improving the mechanical characteristics of the material as a result of processing at ultra-low temperatures. The greatest effect can be achieved when processing such metal products as special cutting, stamping, pressing, rolling, grinding tools, bearings, and critical springs. The basic properties of the metal acquired during deep cooling are retained throughout their entire service life, so re-processing is not required. Cryogenic technology does not replace existing methods of thermal hardening, but makes it possible to impart new properties to the cold-processed material, which ensure maximum use of the resource of the material specified by metallurgists. Using tools treated with ultra-low temperatures allows businesses to significantly reduce costs by:
Read also: Horizontal drilling machine 2р82
The theoretical development and practical development of the cryogenic processing process is considered an achievement of Soviet science. The works of such scientists as G.V. Kurdyumova, the studies of A.P. Gulyaev, V.G. Vorobyov and others are related to cold processing to improve the quality characteristics of hardened steel.
A few years after the publication of the research of Soviet scientists, the first similar works appeared in the foreign press, the authors of which referred to Soviet works as the primary source. It was the work of Soviet scientists that made it possible to fully evaluate the effectiveness of the influence of cold treatment on the properties of steel and laid the foundation for the modern development and use of this processing method. In the 1940-1950s, Soviet industrial enterprises tried to introduce cryogenic processing of tools made from high-speed steels in liquid nitrogen, but this not only did not give the expected result, but also led to a decrease in the strength of the tool, since microcracks appeared due to sudden and uneven cooling . The method allowing the transformation of retained austenite into martensite had to be abandoned, mainly due to economic infeasibility - the high cost of nitrogen as the main refrigerant.
In the USA, Japan, Germany, and South Korea, the topic of cryogenic processing as an effective method for processing structural and tool steels was developed, and decades of research and experience led to the result - currently, cryogenic processing technology is successfully used in many industries.
Metalworking and mechanical engineering:
- increase in tool and equipment life up to 300%
- increasing the wear resistance of materials
- increase in cyclic strength
- increase in corrosion and erosion resistance
- removal of residual stresses
Transport and special equipment:
- increase in brake disc service life up to 250%
- increasing the efficiency of the braking system
- increase in cyclic strength of suspension springs and other elastic elements by 125%
- increase in engine life and power
- reduction in vehicle operating costs
- increase in weapon use up to 200%
- reducing the effect of weapon heating on shooting results
- increasing the service life of components and mechanisms
Mining and manufacturing industry:
- increasing the durability of rock cutting tools up to 200%
- reduction of abrasive wear of machines and mechanisms
- increasing the corrosion and erosion resistance of equipment
- increasing the service life of industrial and mining equipment
Audio equipment and musical instruments:
- reduction of signal distortion in conductors
- reduction of heat dissipated by conductors by 30-40%
- Improved musical detail, clarity and transparency of sound
- expanding the sound range of musical instruments
The use of cryogenic processing is relevant for almost any industry where there is a need to increase service life, increase fatigue strength and wear resistance, and also require increased productivity.
Processing of tool alloys
High, medium and low steel tempering are only suitable for heat treatment of alloys containing less than 0.7% carbon. For alloys with a higher carbon content (called tool alloys), other methods are used. Let's look at the main technologies:
- It is not recommended to temper high-speed tool alloys because they contain molybdenum, cobalt, tungsten, and vanadium. These elements are resistant to heat, so they do not change their physical and chemical properties during tempering heating. Instead of tempering, it is recommended to do multi-stage hardening: for this, the material is gradually heated to 800, 1050 and 1200 degrees - after which the alloy is sharply cooled in an oil environment.
- It is recommended to process conventional tool alloys in two stages. First, the material is hardened in molten salts at a temperature of 450-500 degrees. After this, the second stage is performed - double tempering at a temperature of 550-600 degrees (no more than 1 hour). Please note that when tool alloys are heated, the possibility of the occurrence of tempering ability of the second type is excluded.
Examples [edit | edit code]
Homogenization annealing + aging
For example, for nickel-based superalloys (such as Inconel 718), the following heat treatment is typical: Structure homogenization and dissolution of inclusions (Solution Heat Treatment) at 768-782 °C with accelerated cooling.
Then two-stage aging is carried out (English Precipitation Heat Treatment) - 8 hours at a temperature of 718 ° C, slow cooling for 2 hours to 621-649 ° C and holding for 8 hours. This is followed by accelerated cooling. Quenching + high tempering (improvement)
Many steels are hardened by quenching - accelerated cooling (in air, oil or water). Rapid cooling usually leads to the formation of a nonequilibrium martensitic structure. Steel immediately after hardening is characterized by high hardness, residual stresses, low ductility and toughness. Thus, steel 40ХНМА (SAE 4340) immediately after hardening has a hardness above 50 HRC; in this state, the material is unsuitable for further use due to its high tendency to brittle fracture. Subsequent tempering - heating to 450 °C - 500 °C and holding at this temperature leads to a decrease in internal stresses due to the decomposition of hardened martensite, reducing the degree of tetragonality of its crystal lattice (transition to tempered martensite). At the same time, the hardness of the steel decreases slightly (to 45 - 48 HRC). Steels with a carbon content of 0.3 - 0.6% C are subjected to improvement.
Heat treatment of steel allows you to give products, parts and workpieces the required qualities and characteristics. Depending on the stage at which heat treatment was carried out in the manufacturing process, the workpieces’ workability increases, residual stresses are removed from the parts, and the parts’ performance qualities increase.
Steel heat treatment technology is a set of processes: heating, holding and cooling with the aim of changing the internal structure of the metal or alloy. In this case, the chemical composition does not change.
Thus, the molecular lattice of carbon steel at a temperature of no more than 910°C is a body-centered cube. When heated above 910°C to 1400°C, the lattice takes the shape of a face-centered cube. Further heating turns the cube into a body-centered one.
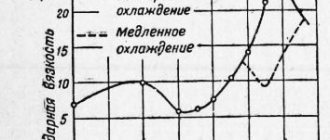
The essence of heat treatment of steels is a change in the grain size of the internal structure of the steel. Strict adherence to temperature conditions, time and speed at all stages, which directly depend on the amount of carbon, alloying elements and impurities that reduce the quality of the material. During heating, structural changes occur, which upon cooling occur in the reverse order. The figure shows what transformations occur during heat treatment.
Change in metal structure during heat treatment
Features of heat treatment of non-ferrous alloys
Most alloys can be subjected to two types of heat treatment - hardening and aging. The last type is a tempering carried out at temperatures of 120...2000C, with cooling at room temperature (natural aging) or with the supply of an air stream (artificial aging).
However, there is a large scatter between many combinations of metals and non-ferrous alloys in the rate of strain hardening, which makes it difficult to systematize the heat treatment processes of non-ferrous alloys.
Key Features:
- Alloys of the copper-nickel system are effectively amenable to mechanical-thermal treatment, during which the structure becomes fine-grained, but the hardness increases.
- All types of non-ferrous alloys can be annealed, and the type of heating does not matter, since the intensity of scale formation is low. Time has less influence on the efficiency of annealing than temperature.
- Hardening of non-ferrous alloys is much less effective. With the exception of titanium, the commonly used alloys of aluminum, copper and magnesium are not allotropic; thus, they do not react in the same way as steel when they are heated and cooled.
- Many alloys such as bronzes cannot be heat treated at all, since for these alloys the solid solutions formed at elevated temperatures remain completely stable at room temperature or below.
- Heat treatment temperature and time cycles cover a wide range, which depends not only on the composition of the alloy, but also on whether the alloy is in a wrought or cast state.
Non-ferrous metals are rarely preheated because it increases the grain size and degrades the alloy structure.
Purpose of heat treatment
Heat treatment of steel is carried out at temperatures close to critical points. Here's what happens:
- secondary crystallization of the alloy;
- transition of gamma iron to the alpha iron state;
- transition of large particles into plates.
Read also: Name of wood cutters
The internal structure of a two-phase mixture directly affects performance and ease of processing.
Formation of structures depending on cooling intensity
The main purpose of heat treatment is to give steels:
- In finished products:
- strength;
- wear resistance;
- corrosion resistance;
- heat resistance.
- In blanks:
- relief of internal stress after
- casting;
- stamping (hot, cold);
- deep drawing;
Heat treatment is applied to the following types of steels:
- Carbon and alloyed.
- With varying carbon contents, from low carbon 0.25% to high carbon 0.7%.
- Structural, special, instrumental.
- Any quality.
Modes of medium (medium temperature) tempering of steel
The temperatures of the medium-temperature tempering process are +350…+500°C. This type of t/o, used mainly for springs, leaf springs, stamps, provides significant limits of endurance and elasticity, and good relaxation resistance. The resulting structures: troostite or trostomartensite, hardness – 45-50 HRC.
Cooling in water after heating to temperatures of +400...+450°C is used for springs in order to create residual compressive stresses on the surface, increasing the strength characteristics of the metal.
Classification and types of heat treatment
The fundamental parameters affecting the quality of heat treatment are:
- heating time (speed);
- heating temperature;
- duration of holding at a given temperature;
- cooling time (intensity).
By changing these modes, you can obtain several types of heat treatment.
Types of heat treatment of steel:
- Annealing
- I – kind:
- homogenization;
- recrystallization;
- isothermal;
- removal of internal and residual stresses;
- Hardening;
- Vacation:
- short;
- average;
- high.
- Normalization.
Heating temperature of steel during heat treatment
Vacation
Tempering in mechanical engineering is used to reduce the strength of internal stresses that appear during hardening. High hardness makes products brittle, so tempering is used to increase impact strength and reduce the hardness and brittleness of steel.
Vacation low
Low tempering is characterized by the internal structure of martensite, which, without reducing hardness, increases viscosity. Measuring and cutting tools are subjected to this heat treatment. Processing modes:
- Heating to a temperature of 150°C, but not higher than 250°C;
- holding time - one and a half hours;
- cooling - air, oil.
Average holiday
For medium tempering, transformation of martensite into trostite. Hardness decreases to 400 HB. Viscosity increases. Parts that operate under significant elastic loads are subjected to this tempering. Processing modes:
- heating to a temperature of 340°C, but not higher than 500°C;
- cooling - air.
High holiday
With high tempering, sorbitol crystallizes, which eliminates stress in the crystal lattice. Critical parts are manufactured that have strength, ductility, and toughness.
Heating to a temperature of 450°C, but not higher than 650°C.
Annealing
The use of annealing makes it possible to obtain a homogeneous internal structure without stress on the crystal lattice. The process is carried out in the following sequence:
- heating to a temperature slightly above the critical point, depending on the grade of steel;
- holding with constant temperature maintenance;
- slow cooling (usually cooling occurs together with the furnace).
Homogenization
Homogenization, otherwise known as diffusion annealing, restores the non-uniform segregation of castings. Processing modes:
- heating to a temperature from 1000°C, but not higher than 1150°C;
- exposure – 8-15 hours;
- cooling:
- oven – up to 8 hours, temperature reduction to 800°C;
- air.
Recrystallization
Recrystallization, otherwise low annealing, is used after plastic deformation treatment, which causes hardening by changing the grain shape (hardening). Processing modes:
- heating to a temperature above the crystallization point by 100°C-200°C;
- holding – ½ – 2 hours;
- cooling is slow.
Isothermal annealing
Alloy steels are subjected to isothermal annealing to cause austenite decomposition. Heat treatment modes:
- heating to a temperature of 20°C - 30°C above the point;
- holding;
- cooling:
- fast - not lower than 630°C;
- slow – at positive temperatures.
Stress Relief Annealing
Removal of internal and residual stresses by annealing is used after welding, casting, and machining. With the application of work loads, parts are subject to destruction. Processing modes:
- heating to a temperature of – 727°C;
- holding - up to 20 hours at a temperature of 600°C - 700°C;
- cooling is slow.
Complete annealing
Full annealing makes it possible to obtain an internal structure with fine grains, which contains ferrite and pearlite. Full annealing is used for cast, forged and stamped workpieces, which will subsequently be processed by cutting and subjected to hardening.
Complete annealing of steel
- heating temperature – 30°C-50°C above point ;
- excerpt;
- cooling to 500°C:
- carbon steel – temperature decrease per hour is no more than 150°C;
- alloy steel – temperature decrease per hour is no more than 50°C.
Partial annealing
With incomplete annealing, lamellar or coarse pearlite is transformed into a ferrite-cementite grain structure, which is necessary for welds produced by electric arc welding, as well as tool steels and steel parts subjected to processing methods whose temperature does not provoke grain growth of the internal structure.
- heating to a temperature above the point or, above 700°C by 40°C - 50°C;
- curing - about 20 hours;
- cooling is slow.
Hardening
Hardening of steels is used for:
- Promotions:
- hardness;
- strength;
- wear resistance;
- elastic limit;
- Reductions:
- plasticity;
- shear modulus;
- compression limit.
The essence of hardening is the fastest cooling of a thoroughly heated part in various environments. Heating is performed with and without polymorphic changes. Polymorphic changes are possible only in those steels that contain elements capable of transformation.
Such an alloy is heated to a temperature at which the crystal lattice of the polymorphic element undergoes changes, due to which the solubility of alloying materials increases. As the temperature decreases, the lattice changes structure due to an excess of alloying element and takes on a needle-like structure.
The impossibility of polymorphic changes during heating is due to the limited solubility of one component in another at a rapid cooling rate. There is little time for diffusion. The result is a solution with an excess of undissolved component (metastable).
To increase the cooling rate of steel, the following media are used:
- water;
- water-based brine solutions;
- technical oil;
- inert gases.
Read also: Lubricant for the patriot cultivator gearbox
Comparing the rate of cooling of steel products in air, cooling in water from 600°C occurs six times faster, and from 200°C in oil 28 times faster. Dissolved salts increase the hardening ability. The disadvantage of using water is the appearance of cracks in places where martensite forms. Industrial oil is used to harden alloy alloys, but it sticks to the surface.
Metals used in the manufacture of medical products should not have a film of oxides, so cooling occurs in an environment of rarefied air.
To completely get rid of austenite, which causes high brittleness in steel, products are subjected to additional cooling at temperatures from -40°C to -100°C in a special chamber. You can also use carbonic acid mixed with acetone. This processing increases the accuracy of parts, their hardness, and magnetic properties.
If parts do not require volumetric heat treatment, only the surface layer is heated using HDF (high-frequency current) installations. In this case, the depth of heat treatment ranges from 1 mm to 10 mm, and cooling occurs in air. As a result, the surface layer becomes wear-resistant, and the middle is viscous.
The hardening process involves heating and holding steel products at temperatures reaching about 900°C. At this temperature, steels with a carbon content of up to 0.7% have a martensite structure, which, during subsequent heat treatment, will transform into the required structure with the appearance of the desired qualities.
Normalization
Normalization produces a fine grain structure. For low-carbon steels this is a ferrite-pearlite structure, for alloyed steels it is a sorbitol-like structure. The resulting hardness does not exceed 300 HB. Hot-rolled steels are subjected to normalization. At the same time, they increase:
- fracture resistance;
- processing performance;
- strength;
- viscosity.
Steel normalization process
- heating occurs to a temperature of 30°C-50°C above the point ;
- maintaining in a given temperature range;
- cooling - in the open air.
Equipment used
Thermal furnaces can be divided into two main types: batch and continuous. The fundamental difference between them lies in how the workpieces being processed are placed in the units, and how they interact with the atmosphere inside the furnaces.
The main energy sources for heating equipment are natural gas and electricity. Alternative energy sources, such as fuel oil, are used less frequently.
Furnaces in which metals are heat treated are classified according to the upper limit of heating temperature. The commonly used temperature range is from 600 to 8000C. Convection heating is mainly used, based on the circulation of air, combustion products or inert gas located inside the furnace.
Batch installations, as a rule, process workpieces in batches, and the heating of each batch can last several hours (and sometimes even days). In a batch kiln, the workload is usually stationary so that it interacts with changes in the kiln atmosphere under near-equilibrium conditions. Types of batch furnaces:
- Bell-shaped;
- Box-shaped;
- Heating wells;
- With a movable hearth;
- Fluidized bed;
- Mine;
- Vacuum.
Continuous furnaces differ in the method of movement of the processed workpieces and the characteristics of the working environment (air, inert gas or vacuum).
Types of continuous furnaces:
- Chamber;
- Tape
- Monorails
- Push
- With roller/rotating hearth;
- Vibrating hearth furnaces;
- Vacuum ovens;
- With walking beams.
The best control of heating parameters is provided by electric furnaces.
Benefits of Heat Treatment
Heat treatment of steel is a technological process that has become a mandatory step in obtaining sets of parts made of steel and alloys with specified qualities. This can be achieved by a wide variety of modes and methods of thermal exposure. Heat treatment is used not only for steels, but also for non-ferrous metals and alloys based on them.
Steels without heat treatment are used only for the construction of metal structures and the manufacture of non-critical parts, the service life of which is short. There are no additional requirements for them. Everyday operation, on the contrary, dictates stricter requirements, which is why the use of heat treatment is preferable.
In thermally untreated steels, abrasive wear is high and proportional to its own hardness, which depends on the composition of chemical elements. Thus, non-hardened die matrices are well combined when working with hardened punches.
If you find an error, please select a piece of text and press Ctrl+Enter.
Is it possible to temper steel at home?
Most often, heat treatment applies to various simple parts, household utensils - knives, forks, metal cups, car parts, and so on. However, home metallurgy has many limitations that the common man may not be aware of. Let's consider the main problems that a person may encounter during a steel holiday at home:
- Most home ovens cannot reach high temperatures. Therefore, at home you can only make a low or medium vacation. Theoretically, you can try to re-equip or “strengthen” your stove to increase the heating temperature, but this will be difficult for a person without experience.
- To carry out heat treatment, it is necessary to use a protective medium (oil, alkali, saltpeter). But each substance has its own temperature characteristics. A simple example: saltpeter-based compounds can explode when heated to high temperatures, which can be dangerous to the life and health of the home metallurgist.
- Tempering without the use of a protective environment can be fatal to the metal itself. The fact is that without the use of a protective environment, the metal will cool quickly, which can affect the quality of the steel (increased brittleness, bending, plastic deformation, rust).
- Also, do not forget about low-temperature brittleness of the first type (from 250 to 300 degrees). If the temperature is incorrect, the quality of the metal can be seriously affected, up to the complete destruction of the alloy.
Pre-heat treatment
Preliminary heat treatment (PHT) is intended to solve technological problems, which in mass mechanical engineering include: improving machinability by cutting or cold deformation, relieving internal stresses, eliminating defects resulting from fire cleaning, electrophysical or laser processing, etc. In addition, PHE is used to improve properties after final heat treatment.
The main technological operations of PHE are annealing and normalization.