- Hardening
- Heating the metal
- Product protection from scale and decarburization
- Coolants
- Vacation process
Heat treatment of steels is one of the most important operations in mechanical engineering, the correct implementation of which determines the quality of the products. Quenching and tempering of steels are one of the various types of heat treatment of metals.
Thermal effects on metal change its properties and structure. This makes it possible to increase the mechanical properties of the material, the durability and reliability of products, as well as reduce the size and weight of mechanisms and machines. In addition, thanks to heat treatment, cheaper alloys can be used for the manufacture of various parts.
It also won’t hurt you to know how to cook with a semi-automatic machine.
As the Steel Was Tempered
Heat treatment of steel involves applying heat to the metal under certain conditions to change its structure and properties.
Heat treatment operations include:
- annealing;
- normalization;
- aging;
- steel hardening and steel tempering (etc.).
Heat treatment of steel: hardening, tempering - depends on the following factors:
- heating temperatures;
- heating time (speed);
- duration of exposure at a given temperature;
- cooling rate.
Hardening
Steel hardening is a heat treatment process, the essence of which is to heat steel to a temperature above the critical temperature, followed by rapid cooling. As a result of this operation, the hardness and strength of steel increase, and ductility decreases.
When steels are heated and cooled, the atomic lattice is rearranged. The critical temperature values for different grades of steel are not the same: they depend on the content of carbon and alloying impurities, as well as on the rate of heating and cooling.
After hardening, the steel becomes brittle and hard. When heated in thermal furnaces, the surface layer of products becomes covered with scale and is decarbonized the more, the higher the heating temperature and the holding time in the furnace. If the parts have a small allowance for further processing, then this defect is irreparable. Hardening modes for hardening steel depend on its composition and technical requirements for the product.
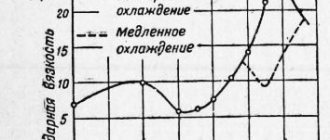
During hardening, parts should be cooled quickly so that austenite does not have time to transform into intermediate structures (sorbitol or troostite). The required cooling rate is ensured by selecting the cooling medium. In this case, excessively rapid cooling leads to cracks or warping of the product. To avoid this, in the temperature range from 300 to 200 degrees, the cooling rate must be slowed down, using combined hardening methods. The method of immersing the part in a cooling medium is of great importance to reduce warping of the product.
Heating the metal
All steel hardening methods consist of:
- heating steel;
- subsequent holding to achieve through-heating of the product and completion of structural transformations;
- cooling at a certain speed.
Carbon steel products are heated in chamber furnaces. In this case, preheating is not required, since these steel grades are not subject to cracking or warping.
Complex products (for example, a tool with protruding thin edges or sharp transitions) are preheated:
- in salt baths by immersing two or three times for 2 - 4 seconds;
- in separate ovens up to a temperature of 400 - 500 degrees Celsius.
Heating of all parts of the product should proceed evenly. If this cannot be achieved in one step (large forgings), then two holding times are made for through heating.
If only one part is placed in the oven, the heating time is reduced. For example, one 24 mm thick disk cutter heats up within 13 minutes, and ten such products heat up within 18 minutes.
Vickers method
When measuring hardness using the Vickers method, a pyramid-shaped tip is used as an indenter, the edges of which converge at an angle of 136 degrees. To ensure the accuracy of the test, it is important to observe several points:
- the load must be placed strictly in the center of the diamond tip;
- the load application vector must be strictly perpendicular to the surface of the test sample.
Measurements take place according to the following algorithm: the sample being tested is placed on a special table, and the indenter is pressed into the sample from above immediately with the required load level (the maximum possible value is up to 100 kgf). Next, the indenter is held under load for 10-15 seconds. After removing the indenter, the indentation depth and indentation diagonal are measured.
Next, a calculation takes place according to the form, which takes into account the ratio of the applied load to the diagonal of the indentation and the time during which the test took place. Hardness is indicated in kgf/mm2 format, display format HV. The Vickers method, due to the use of a diamond tip, allows for more accurate measurements than the Brinell method.
Product protection from scale and decarburization
For products whose surfaces are not ground after heat treatment, carbon burnout and scale formation are unacceptable. Surfaces are protected from such defects by using protective gases supplied into the cavity of the electric furnace. Of course, this technique is only possible in special sealed ovens. The source of gas supplied to the heating zone is shielding gas generators. They can operate on methane, ammonia and other hydrocarbon gases.
If there is no protective atmosphere, then before heating the products are packaged in containers and covered with used carburizer and cast iron shavings (the heat engineer should know that charcoal does not protect tool steels from decarburization). To prevent air from getting into the container, it is coated with clay.
When heated, salt baths prevent the metal from oxidizing, but do not protect against decarbonization. Therefore, in production they are deoxidized at least twice per shift with brown salt, blood salt or boric acid. Salt baths operating at temperatures of 760 – 1000 degrees Celsius are very effectively deoxidized by charcoal. To do this, a glass with many holes over the entire surface is filled with dried charcoal, closed with a lid (so that the coal does not float up) and, after heating, lowered to the bottom of the salt bath. First, a significant number of flames appear, then it decreases. If you deoxidize the bath three times during a shift in this way, the heated products will be completely protected from decarbonization.
The degree of deoxidation of salt baths is checked very simply: an ordinary blade, heated in a bath for 5 - 7 minutes in a high-quality deoxidized bath and hardened in water, will break, not bend.
Coolants
The main coolant for steel is water. If you add a small amount of salts or soap to the water, the cooling rate will change. Therefore, under no circumstances should the quenching tank be used for other purposes (for example, washing hands). To achieve the same hardness on the hardened surface, it is necessary to maintain the coolant temperature at 20 - 30 degrees. You should not change the water in the tank frequently. It is absolutely unacceptable to cool the product in running water.
The disadvantage of water hardening is the formation of cracks and warping. Therefore, only products of simple shapes or cemented ones are hardened using this method.
- When hardening products of complex configurations made of structural steel, a fifty percent solution of caustic soda is used (cold or heated to 50 - 60 degrees). Parts heated in a salt bath and hardened in this solution turn out light. The solution temperature should not be allowed to exceed 60 degrees.
Modes
Vapors formed during quenching in a caustic solution are harmful to humans, so the quenching bath must be equipped with exhaust ventilation.
- Alloy steel is hardened in mineral oils. By the way, thin carbon steel products are also carried out in oil. The main advantage of oil baths is that the cooling rate does not depend on the oil temperature: at a temperature of 20 degrees and 150 degrees, the product will cool at the same rate.
Be careful not to let water get into the oil bath, as this may cause the product to crack. What is interesting: in oil heated to a temperature above 100 degrees, the ingress of water does not lead to the appearance of cracks in the metal.
The disadvantage of an oil bath is:
- release of harmful gases during hardening;
- formation of plaque on the product;
- oil's tendency to flammability;
- gradual deterioration of hardening ability.
- Steels with stable austenite (for example, X12M) can be cooled with air supplied by a compressor or fan. At the same time, it is important to prevent water from entering the air duct: this can lead to the formation of cracks in the product.
- Step hardening is performed in hot oil, molten alkalis, and low-melting salts.
- Intermittent hardening of steels in two cooling environments is used for processing complex parts made of carbon steels. First they are cooled in water to a temperature of 250 - 200 degrees, and then in oil. The product is kept in water for no more than 1 - 2 seconds for every 5 - 6 mm of thickness. If the exposure time in water is increased, cracks will inevitably appear on the product. Transferring the part from water to oil must be done very quickly.
Welding a car yourself is not an easy task, but it can be done. Do you need to cut metal quickly and efficiently? Use a plasma cutter! How to do it correctly, read this article.
If you are interested in how to turn metal products, read the article at https://elsvarkin.ru/obrabotka-metalla/tokarnaya-obrabotka-metalla-obshhie-svedeniya/ link.
Application of U10 steel
Alloys U10 and U10A are used for the manufacture of tools and devices that experience loads without overheating the tip. Such tools include taps for cutting internal threads, wood saw blades, rasps, axes, and cold-action die stampers.
Cold-rolled steel strip is used for the manufacture of various springs, probes, valves, bifurcating blades, lamella contacts, and parts for the watch industry. The thickness of the tape ranges from 20 microns to 2.5 mm, the difference is quite noticeable.
U10 steel is used to make saw blades for drives, cores, cross saws, needle wire, conventional drilling cutters and cold forming dies. Simplified gauges, scrapers, rolling rollers and files are also produced from the alloy.
Alloy U10A is used for the manufacture of knives for general utility and special purposes. They are used in home life and catering establishments. The stamping of U10 steel should be no wider than 3 cm, then the product will be of decent quality.
Examples of knives made of steel U10 and U10A.
Vacation process
All hardened parts are subject to tempering. This is done to relieve internal stress. As a result of tempering, the hardness of the steel is slightly reduced and the ductility of the steel is increased.
Depending on the required temperature, tempering is carried out:
- in oil baths;
- in saltpeter baths;
- in furnaces with forced air circulation;
- in baths with molten alkali.
The tempering temperature depends on the grade of steel and the required hardness of the product, for example, a tool that requires a hardness of HRC 59 - 60 should be tempered at a temperature of 150 - 200 degrees. In this case, internal stresses decrease and hardness decreases slightly.
High-speed steel is tempered at a temperature of 540 - 580 degrees. This tempering is called secondary hardening, since as a result the hardness of the product increases.
Products can be tarnished by heating them on electric stoves, in ovens, even in hot sand. The oxide film that appears as a result of heating acquires different tarnish colors, depending on the temperature. Before you start tempering one of the tarnish colors, you need to clean the surface of the product from scale, oil deposits, etc.
Usually, after tempering, the metal is cooled in air. But chromium-nickel steels should be cooled in water or oil, since slow cooling of these grades leads to temper brittleness.
Analogs
Substitutes for the alloy can be called Russian steel grades U11 and U12. According to the markings, it becomes clear that the alloys contain slightly more carbon. Oddly enough, U10A steel has quite a lot of analogues. Let's look at them in more detail:
- Hungarian: S-101/102;
- Italian: ABN/C-100-KN;
- Spanish: C-102;
- French: C-105-E2U;
- British alloy: 1645;
- Romanian: OSC-10;
- Swedish: 1880;
- Czechoslovakian: 19191;
- Pan-European: AFNOR-NF;
- Japanese: SK-3;
- American: T-72301;
- Spanish: F. 515;
- Polish: N-10 (E);
- Chinese: T10 (A).