Weldability groups of steels
Each metal can be classified into several weldability groups. There are 4 steel weldability groups in total.
1 group . This group is characterized by good metal weldability. Welding of such metal is carried out without heating and without subsequent heat treatment. Although in some cases it is possible to slightly heat the steel after welding. This will help relieve internal tension.
2nd group . Steels of this group are prone to the formation of small defects (cracks) during the welding process. To prevent this, it is necessary to preheat the metal to 120-150°C.
3 group . Steels of this group are difficult to weld. Partial detachment of the seam from the base metal occurs. Cracks and other defects may form. To perform welding, heating to 300-500°C and subsequent smooth cooling is required. This is the only way to achieve an acceptable result.
4 group . This is the worst group of steel weldability. Such steels are not used for the manufacture of welded structures. They are used to manufacture parts by mechanical processing.
Therefore, when choosing a metal for the manufacture of various welded structures, you must first decide on the grade of steel. After this, in V. G. Sorokin’s steel grader you can find out about the weldability of steel. You can download the guide from the link above. But you can calculate the weldability yourself.
Low alloy steels
Low-alloy steels must have good ductility, satisfactory weldability and high resistance to brittle fracture. They acquire optimal mechanical properties after hardening or normalization and subsequent high tempering. Examples of low-alloy steels are 14G2, 14KhGS, 15GS and others. They are characterized by low carbon content (<0.18%). High mechanical properties of low-alloy and low-carbon steels are achieved by using other additives (manganese, chromium, nickel, silicon and others).
These types of metal are characterized by good weldability and good impact strength with a low cold brittleness limit (- 40C° - - 60C°). They have a fine-grained structure, as they are produced calm. The presence of nickel, chromium, and copper increases the corrosion resistance of many grades of steel. However, low-alloy materials have increased sensitivity to stress concentration and therefore have lower vibration strength.
Welding technology for low-alloy metals
The main indicators of the weldability of low-alloy steels are the resistance of welded joints to cold cracks and brittle fracture. Such metals usually have a limited content of C, Ni, Si, S and P, so if welding conditions are observed and filler materials are used correctly, hot cracks will not occur. The criteria for determining the range of welding modes and preheating temperatures are the permissible maximum and minimum cooling rates of the metal in the heat-affected zone. The maximum permissible cooling rates are adopted in such a way as to prevent the formation of cold cracks in the metal of the heat-affected zone.
Chemical composition of alloys
Electrodes for welding low-alloy steels using manual arc welding have a low-hydrogen calcium fluoride coating. Electrodes of type E70 according to GOST 9467-75 are widely used. Welding is performed with direct current with reverse polarity. The metal deposited with electrodes must correspond to the following chemical composition,%: C up to 0.10; Mn 0.8…1.2 ; Si 0.2…0.4 ; Cr 0.6…1.0 ; Mo 0.2…0.4 ; Ni 1.3…1.8 ; S up to 0.03; P up to 0.03. The welding current is selected depending on the brand and diameter of the electrode, taking into account the position of the seam in space, the type of connection and the thickness of the metal being welded. Welding of technological sections must be carried out without interruptions, preventing the welded joint from cooling below the preheating temperature and heating it above 200C° before performing the next pass.
Features of submerged arc welding of low-alloy steels are that it is carried out using direct current of reverse polarity. The current strength should not exceed 800 A, the arc voltage should not exceed 40 V, and the welding speed should be varied within 13...30 m/h. Single-sided single-pass welding is used for joints up to 8 mm thick and is performed on the remaining steel backing or flux pad. The maximum thickness of joints without cutting edges, welded with double-sided seams, should not exceed 20 mm. For butt joints without beveled edges (one-sided or double-sided), wire of the Sv-08KhN2M grade is used, since the seams in this case have excessively high strength and the use of more alloyed wire for such connections is impractical.
The influence of alloying elements on the structure and properties of metals
If welding of low-carbon and low-alloy steels is carried out in carbon dioxide, then wire of the Sv-08G2S, Sv-10KhG2SMA, Sv-08KhN2G2SMYu grades (GOST 2246-70) or flux-cored wire is used as an electrode. When welding in argon-based mixtures, wire of the Sv-08KhN2GMYu brand is used, which provides a high level of mechanical properties and cold resistance of metal welds when welding steels with a strength of up to 700 MPa. Wires of the specified grades are also recommended for welding fillet welds with a leg over 15 mm. For fillet welds with a smaller leg, in most cases, wire of the Sv-08G2S grade is used. This wire is also used for welding low-carbon and low-alloy high-strength steels 09G2, 10G2S1, 14G2, 10KhSND and 15KhSND.
Gas welding of low-alloy steels is characterized by increased heating of the welded edges, reduced corrosion resistance and increased burnout of alloying impurities. This leads to deterioration in the quality of welded joints compared to other welding methods. When gas welding, wire of the SV-10G2, Sv-08, Sv-08A grades is used as a filler material, and for critical welds - Sv-18KhGS and Sv-18KhMA. The mechanical properties of the seam can be increased by forging at a temperature of 800 ° C - 850 ° C with subsequent normalization.
Determination of steel weldability by carbon equivalent
To determine the weldability of steel, it is necessary to calculate the carbon equivalent (Ceq). The weldability of the metal directly depends on carbon. Therefore, the carbon coefficient is an important indicator for determining the weldability of steel.
You can calculate it using this formula:
Sek = C + Mn/6 + Cr + Mo/5 + V + (Ni + Cu)/15 ,
where: C is carbon content in percent, %;
Mn —manganese content in percent, %;
Cr —chromium content in percent, %;
Mo —molybdenum content in percent, %;
V —vanadium content in percent, %;
Ni —nickel content in percent, %;
Cu —copper content in percentage, %.
Knowing the CEC of steel, it is possible to determine which weldability group the steel belongs to.
Sack up to 0.25% - 1 group (Welded without restrictions);
SEC over 0.25 to 0.35% - group 2 (Limited weldability);
SEC over 0.35 to 0.45% - group 3 (Difficult to weld);
SEC over 0.45% - group 4 (Not used for welding).
Classification of steels by weldability
When classifying steels according to weldability, they can be divided into the following groups:
1. Can be welded without restrictions (good);
2. Limited weldability (satisfactory);
3. Difficult to weld (limited);
4. Not suitable for welding (poor).
Medium alloy steels
Medium alloy steels contain carbon in amounts of 0.4% or more. They are alloyed mainly with Ni, Mo, Cr, V, W. The optimal combination of strength, toughness and ductility is achieved after hardening and low tempering. Medium alloy steels such as KhVG, KhVSG, 9HS are in great demand due to their alloying additives in the manufacture of drills, reamers and broaches.
These steels are smelted from pure charge materials to increase ductility and toughness. They are also carefully cleaned from phosphorus, sulfur, gases and various non-metallic inclusions. In this case, steel can be subjected to electroslag or vacuum-arc remelting, or refining in a ladle with liquid synthetic slag. A good combination of strength, toughness and ductility of medium-alloy steels is achieved by thermomechanical processing.
Welding technology for medium alloy metals
To ensure the operational reliability of welded joints, when choosing welding materials, it is necessary to strive to obtain welds of such a chemical composition that their mechanical properties would have the required values. degree of change in these properties depends on the share of participation of the base metal in the formation of the weld. Therefore, you should choose welding materials that contain less alloying elements than the base metal. Alloying the weld metal at the expense of the base metal makes it possible to increase the properties of the weld to the required level.
When welding medium-alloy, deeply hardening, high-strength steels, it is necessary to select welding materials that will ensure the production of welds with high deformation capacity with the minimum possible amount of hydrogen in the weld pool. This is achieved by using low-alloy welding electrodes that do not contain organic substances in the coating and are subjected to high-temperature calcination. At the same time, when performing welding work, other sources of saturation of the weld pool with hydrogen (moisture, rust, etc.) should be excluded.
Austenitic welding consumables are widely used for welding medium-alloy steels. For mechanized welding and the manufacture of electrode rods, GOST 2246-70 provides for wire grades Sv-08Х20Н9Г7Т and Sv-08Х21Н10Г6, and GOST 10052-75 - electrodes of type EA-1G6, etc. Electrode coatings are used of type F, and for mechanized welding - basic fluxes. For welding medium-alloyed high-strength steels, electrodes of types E-13X25N18, E-08X21N10G6 and others are used in accordance with GOST 10052-75 and GOST 9467-75.
High quality welded joints with a thickness of 3...5 mm are achieved using argon arc welding with a non-consumable electrode. At the same time, to increase the penetrating ability of the arc, activating fluxes (AF) are used. Welding with AF is effective with mechanized methods to obtain a uniform penetration depth. The non-consumable electrode when welding with AF is selected from the most resistant grades of activated tungsten.
Gas welding of alloy steels is carried out with oxygen acetylene, which provides a high-quality weld. Substitute gases are not recommended in this case. But even oxygen acetylene cannot completely guarantee a high-quality seam. This can only be achieved by using arc welding.
Do-it-yourself metal doors are the ideal solution if you want to save money. Steel hardening is an obligatory stage in mechanical engineering, since the quality of the product depends on the correctness of its implementation. Read more in this article.
You can make very beautiful products from metal. You will find interesting ideas at https://elsvarkin.ru/prakticheskoe-primenenie/suveniry-i-ukrasheniya-iz-metalla-svoimi-rukami/ link.
Welding stainless steel (stainless steel)
Welding stainless steel has its own distinctive features. From our article you will learn a lot of useful information about this process in a few minutes. In one place we have collected basic data on welding methods and important nuances when carrying out work. Read and apply the acquired knowledge in practice. The Tiberis welding equipment store is always happy to share its secrets with you and is happy to help with practical advice.
Content
- Stainless steel - what kind of material is it?
- Where are the different types of stainless steel used?
- What methods are used to weld stainless steel?
- Features of welding stainless steel or how to avoid defects when welding stainless steel
- What quality stainless steel welding equipment should be like?
- Processing products before welding - what and how to do
- How are stainless steel products processed after welding?
- Features of welding stainless steel with other materials
- conclusions
Stainless steel - what kind of material is it?
At all times, the main enemy of iron products was rust. It can turn the most durable structures into a pile of useless scrap metal. Due to oxidation in the open air, precision instruments become unusable and huge structures are destroyed.
But just over a century ago, people managed to find an excellent rust remover. In 1913, English researcher Harry Brierley created the world's first (according to officially recognized version) stainless steel. It contained 12.8% chromium and 0.24% carbon. Although the first experiments with iron and chromium alloys began to be carried out back in 1820.
Stainless steel has pronounced anti-corrosion properties. Stainless steel acquires these characteristics when certain metals are added to its melt. Most often, chromium, nickel, manganese and molybdenum are used for such purposes.
There are 3 main groups of stainless steel according to their chemical composition:
- Chromium (have increased strength) These are the cheapest types of stainless steel. They are less amenable to processing due to low ductility.
- Chrome-nickel (differ in greater ductility). The most popular and widest group of stainless steel. The addition of nickel stabilizes the alloy structure and gives the steel weak magnetic properties.
- Chromium-manganese-nickel. Adding manganese to the alloy increases strength while maintaining the ductility of steel.
1.3. Weldability and the influence of alloying elements on it.
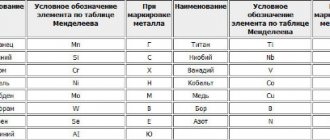
Alloying elements include, first of all,: Cr ,
Ni , Mo , W , V , Ti
, Nb, as well as manganese and silicon at a certain content.
I. Chromium ( Cr )
— low-carbon steels contain up to 0.3%; in structural ones - 0.7-5%, in chromium-nickel ones - 9 - 35%.
“-“
1) When welding, it forms chromium carbides - which sharply increase hardness in the heat-affected zone;
” – ” 2) Promotes the formation of refractory oxides, which complicate the welding process.
II. Nickel ( Ni )
- in low-carbon ones 0.2-0.3%, in alloyed ones 8-35%.
Influence: “±”
1) Increases the plastic and strength properties of steel.
“+ “2) Grinds grain without affecting weldability.
” + ” 1) Increases the load-bearing capacity of steel under shock loads and elevated temperatures.
“-” 2) Promotes the formation of cracks.
“-” 3) Actively oxidizes and burns out.
High alloy steels
High-alloy steels have a higher content of alloying elements - Cr and Ni (usually not lower than 16% and 7%, respectively). They give such metals the appropriate structure and necessary properties. Highly alloyed steels, compared to less alloyed steels, have high cold resistance, corrosion resistance, heat resistance and heat resistance. Despite the high properties of these steels, their main service purpose is determined by the appropriate selection of alloying composition. Accordingly, they can be divided into three groups: heat-resistant, heat-resistant and corrosion-resistant.
After appropriate heat treatment, high-alloy steels have high strength and plastic properties. Unlike carbon materials, when hardened, these materials acquire increased plastic properties.
The structures of high-alloy steels are very diverse and depend mainly on their chemical composition, that is, on the content of the main elements: chromium (ferritizer) and nickel (austenitizer). The structure is also affected by the content of other alloying elements, ferritizers (Mo, Ti, Si, Al, W, V) and austenizers (Co, Cu, C, B).
Welding fluxes - classification
The classification of fluxes is extremely broad. They are distinguished by appearance and physical state, chemical composition, method of production, and purpose. So, for example, for surfacing or arc welding, as a rule, granular or powder fluxes with certain electrical conductivity indicators are used, and for gas welding, gases, powders, and pastes are used.
According to the method of obtaining composites
There are fused and unfused fluxes.
Fused welding flux is widely used not only in welding, but also in surfacing. It demonstrates high efficiency in cases where the surface of the weld metal, by adding additional chemical elements, must obtain higher technical characteristics - for example, increased resistance to corrosion or a very even and smooth weld.
Submerged Surfacing
Fused fluxes are obtained in the following way: the components are ground, mixed, then melted in flame or electric furnaces in the complete absence of oxygen. The heated particles are then passed through a continuous stream of water, hardening and thus turning into granulate. The particle size varies - the thinner the welding rod, the smaller the granules should be.
Unmelted fluxes (ceramic) for welding are made by mixing crushed particles of a mixture of ferroalloys, minerals, and slag-forming materials without subsequent melting. The particles are mixed with glass and then sintered.
Among their advantages:
- low consumption,
- possibility of repeated use,
- high quality of the resulting seam.
IV. Vanadium (V).
Promotes hardening of steel, which makes welding difficult.
It actively oxidizes and burns out.
V. _
Manganese (Mn)
- contained in the range of 0.3-0.8% (for most structural steels).
The welding process is not difficult.
It is an active deoxidizer.
But:
There is a danger of cracks appearing due to the fact that
Mn
increases the hardenability of steel.
Does not cause any difficulties during welding.
When its content is 0.8-1.5%, welding conditions deteriorate due to the high fluidity of silicon steel and the formation of refractory oxides.
Basic criteria establishing weldability
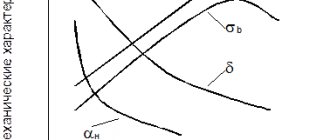
The main indicator of weldability is the carbon equivalent, which is designated as Seq. This conditional coefficient takes into account the level of influence of carbon and alloying components on the properties of the weld.
Factors affecting the weldability of steels:
- Metal sample thickness
- Volume of harmful impurities
- Environmental conditions
- Carbon capacity
- Alloy level
- Microstructure
The main parameter for information is the chemical composition of the material.
Metallurgical processes in gas welding, crystallization of weld metal
During the gas welding process, the molten metal of the weld pool interacts with the welding flame. This interaction is determined by the properties of the metal being welded and the composition of the welding flame. Weld by a reducing flame zone consisting mainly of carbon monoxide and hydrogen. The weld pool is characterized by a small volume of molten metal, high temperature at the welding site and a high rate of melting and crystallization of the metal.
The molten metal of the pool interacts with the gases of the welding flame, resulting in oxidation and reduction reactions. The interaction of gases with different metals is different. Metals that have a high affinity for oxygen are most easily oxidized. Oxidation of the molten metal occurs both due to the oxides located on the surface of the metal being welded and the filler wire, and due to the oxygen of the surrounding air. As the oxygen content in the metal being welded increases, the mechanical properties of the welded joint deteriorate. Therefore, during gas welding for most metals and alloys, special deoxidizers are introduced into filler materials and fluxes to eliminate oxidation processes.