Production
Reference.
Low-carbon steel ST3 is manufactured using open-hearth or oxygen-converter technology. The production method does not affect the parameters of the finished material, however, the oxygen-converter method requires lower financial costs. Steel alloys are made on the basis of ferrite, that is, a solid solution of carbon with alloying elements. This melt is saturated with carbon to increase its strength. The concentration of phosphorus in grade ST3 should not exceed 0.04%, sulfur - 0.05%.
Due to the reaction of ferrite with phosphorus, the ductility of the alloy decreases under the influence of high temperatures, and under the influence of frost the material becomes more brittle. The formation of iron sulfide during the melting process can cause the material to become red brittle.
To improve the performance characteristics of products made from CT3, it is recommended to subject them to heat treatment. Thus, annealing is necessary for complex structures immediately after construction in order to relieve the stresses that arose during welding. Similarly, tension should be removed from parts with a thickness or radius over 36 mm.
Theoretical weight of stainless steel sheets and stainless steel sheets. Density stainless steel
Density of steel of various types and grades: table of temperature dependence of density
| Catalog: Aluminum, duralumin Copper Bronze Brass Tin, babbits, Solder Zinc Lead Titanium Nichrome, fechral Stainless steel Stainless sheet Stainless steel pipe 12Х18Н10Т / AISI 321 / AISI 304 Stainless bends Stainless circle Stainless nickel-containing circle Stainless circle Nickel-free, heat-resistant Stainless, nickel-free, heat-resistant circle Hexagon, nickel-free, stainless heat-resistant Stainless steel hexagon, nickel-containing Stainless mesh Stainless wire Circle 20Х25Н20С2 (ЭИ283) Stainless steel grade Density of stainless steel Stainless fasteners, hardware Electrodes Sale Needs GOSTs and TU Vacancies | K, ρ/7.85 | |
| 08Х22Н6Т | 7,60 | 0,97 |
| 08Х13 | 7,70 | 0,98 |
| 08Х17Т | 7,70 | 0,98 |
| 12Х13 | 7,70 | 0,98 |
| 12Х17 | 7,70 | 0,98 |
| 04Х18Н10 | 7,90 | 1,00 |
| 08Х18Н10 | 7,90 | 1,00 |
| 08Х18Н10Т | 7,90 | 1,00 |
| 08Х20Н14С2 | 7,70 | 0,98 |
| 08Х18Н12Т | 7,95 | 1,01 |
| 08Х18Н12Б | 7,90 | 1,00 |
| 10Х23Н18 | 7,95 | 1,01 |
| 06ХН28МДТ | 7,96 | 1,01 |
| 10Х17Н13М2Т | 8,00 | 1,02 |
| 08Х17Н15М3Т | 8,10 | 1,03 |
Formulas for calculating mass
02Х17Н11М2 20 8000 02Х22Н5АМ3 20 8000 03Н18К9М5Т 20 8000 03Х11Н10М2Т 20 8000 03Х13Н8Д2ТМ (EP699) 20 7800 03Х24Н6AM3 (ЗИ130) 20 8 000 06Х12Н3Д 20 7810 06ХН28МДТ (0Х23Н28М3Д3Т, EI943) 20 7960 07Х16Н6 (Х16Н6, EP288) 20 7800 Steel 08 20…100…200 …300…400…500…600…700…800…900 7871…7846…7814…7781…7745…7708…7668…7628…7598…7602 08GDNFL 20 7850 08kp 20…100…200…300…400…500…6 00 …700…800…900 7871…7846…7814…7781…7745…7708…7668…7628…7598…7602 08Х13 (0Х13, EI496) 20…100…200 7760…7740…7710 08Х17Т (0Х17Т, EI64) 5) 20 7700 08Х17Н13М2Т (0Х17Н13М2Т) 20...100...200...300...400...500...600...700 7900...7870...7830...7790...7750...7700...7660...7620 08Х18Н10 (0Х18Н10) 20 7850 08Х18Н10Т (0Х18 N10T, EI914) 20 7900 08Х22Н6Т (0Х22Н5Т , EP53) 20 7700 3Х3М3Ф 20...100...200...300...400...500...600...700...800...900 7828...7808...7783...7754...7721...7684...7642...7597...7565...7525 4Х4VMFS (DI22) 20… 100…200…300…400…500…600…700…800…900 7808…7786…7757…7726…7693…7658…7624…7581…7554…7550 4Х5МФ1С (EP572) 20…100…200…300…400… 500…600…700…800…900 7716…7692…7660…7627…7593…7559…7523…7490…7459…7438 9ХС 20 7830 9Х2МФ 20 7840 Steel 10 20…100…200…300…400…500 …600… 700...800...900 7856...7832...7800...7765...7730...7692...7653...7613...7582...7594 10G2 20 7790 10kp 20...100...200...300...400...500...600...700...800...900 7856 …7832 …7800…7765…7730…7692…7653…7613…7582…7594 10Х11Н20Т3Р (ЭИ696) 20 7900 10Х11Н23Т3МР (EP33) 20 7950 10Х12Н3М2ФА(Ш) (10Х12Н3М2ФА-А(Ш) ) 20 7750 10Х13Н3М1Л 20 7745 10Х14Г14Н4Т (Х14Г14Н3Т, EI711 ) 20 7800 10Х17Н13М2Т (Х17Н13М2Т, EI448) 20...100...200...300...400...500...600...700 7900...7870...7830...7790...7750...7700...7660...7620 10Х18Н18У4Д (E P841) 20 7630 12МХ 20…100… 200...300...400...500...600...700 7850...7830...7800...7760...7730...7690...7650...7610 12ХН2 20 7880 12ХН3А 20...100...200...300...400...500...600 7850...7830...7 800…7760 ...7720...7680...7640 12X2MFB (EI531) 20 7800 12X1MF (EI575) 20...100...200...300...400...500...600...700...800...900 7800...7780...7750...7720...7680...7650...76 00…7570 ... 7540 ... 7560 12x2N4A 20 ... 100 ... 300 ... 400 ... 600 7840 ... 7820 ... 7760 ... 7710 ... 7630 12x13 (1x13) 20 ... 100 ... 200 ... 300 ... 400 ... 600 ... 700 ... 800 ... 900 7720 ... 7700 ... 7670…7640…7620…7580…7550…7520…7490…7500 12Х17 (Х17, EZh17) 20 7720 12Х18Н9 (Х18Н9) 20…100…200…300…400…500…600…700…800…900 7900… 7860… 7820...7780...7740...7690...7650...7600...7560...7510 12Х18Н9Т (Х18Н9Т) 20...100...200...300...400...500...600...700...800...900 7900...7860...7820...7780...7740...76 90… 7650…7600…7560…7510 12Х18Н10Т 2
pellete.ru
Table of specific gravity of metal alloys
The specific gravity of metals is most often determined in laboratory conditions, but in their pure form they are very rarely used in construction. Alloys of non-ferrous metals and alloys of ferrous metals, which according to their specific gravity are divided into light and heavy, are much more often used.
Light alloys are actively used by modern industry due to their high strength and good high-temperature mechanical properties. The main metals of such alloys are titanium, aluminum, magnesium and beryllium. But alloys based on magnesium and aluminum cannot be used in aggressive environments and at high temperatures.
Heavy alloys are based on copper, tin, zinc, and lead. Among the heavy alloys, bronze (an alloy of copper with aluminum, an alloy of copper with tin, manganese or iron) and brass (an alloy of zinc and copper) are used in many industries. Architectural parts and sanitary fittings are produced from these grades of alloys.
The reference table below shows the main quality characteristics and specific gravity of the most common metal alloys. The list provides data on the density of the main metal alloys at an ambient temperature of 20°C.
| List of metal alloys | Density of alloys (kg/m 3) |
| Admiralty Brass - Admiralty Brass (30% zinc, and 1% tin) | 8525 |
| Aluminum bronze - Aluminum Bronze (3-10% aluminum) | 7700 — 8700 |
| Babbitt – Antifriction metal | 9130 -10600 |
| Beryllium bronze (beryllium copper) - Beryllium Copper | 8100 — 8250 |
| Delta metal - Delta metal | 8600 |
| Yellow Brass - Yellow Brass | 8470 |
| Phosphorous bronzes – Bronze – phosphorous | 8780 — 8920 |
| Regular bronzes - Bronze (8-14% Sn) | 7400 — 8900 |
| Inconel | 8497 |
| Incoloy | 8027 |
| Wrought Iron | 7750 |
| Red brass (low zinc) - Red Brass | 8746 |
| Brass, casting – Brass – casting | 8400 — 8700 |
| Brass, rolled – Brass – rolled and drawn | 8430 — 8730 |
| Light alloys of aluminum – Light alloy based on Al | 2560 — 2800 |
| Light alloys of magnesium – Light alloy based on Mg | 1760 — 1870 |
| Manganese Bronze | 8359 |
| Cupronickel – Cupronickel | 8940 |
| Monel | 8360 — 8840 |
| Stainless Steel | 7480 — 8000 |
| Nickel silver – Nickel silver | 8400 — 8900 |
| Solder 50% tin / 50% lead - Solder 50/50 Sn Pb | 8885 |
| Light antifriction alloy for casting bearings = matte containing 72-78% Cu - White metal | 7100 |
| Lead bronze, Bronze - lead | 7700 — 8700 |
| Carbon steel | 7850 |
| Hastelloy | 9245 |
| Cast iron | 6800 — 7800 |
| Electrum (gold-silver alloy, 20% Au) - Electrum | 8400 — 8900 |
The density of metals and alloys presented in the table will help you calculate the weight of the product. The method for calculating the mass of a part is to calculate its volume, which is then multiplied by the density of the material from which it is made. Density is the mass of one cubic centimeter or cubic meter of a metal or alloy. Mass values calculated on a calculator using formulas may differ from real ones by several percent. This is not because the formulas are not accurate, but because in life everything is a little more complicated than in mathematics: right angles are not quite right, circles and spheres are not ideal, deformation of the workpiece during bending, embossing and hammering leads to unevenness of its thickness , and you can list a bunch more deviations from the ideal. The final blow to our desire for precision comes from grinding and polishing, which lead to unpredictable weight loss in the product. Therefore, the obtained values should be treated as indicative.
DEFINITION
Free-flowing aluminum
is a silvery-white (Fig. 1) light metal. It is easily drawn into wire and rolled into thin sheets.
At room temperature, aluminum does not change in air, but only because its surface is covered with a thin film of oxide, which has a very strong protective effect.
Rice. 1. Aluminum. Appearance.
Aluminum is characterized by high ductility and high electrical conductivity, approximately 0.6 of the electrical conductivity of copper. This is due to its use in the production of electrical wires (which, with a cross-section that ensures equal electrical conductivity, are half the weight of copper). The most important aluminum constants are presented in the table below:
Table 1. Physical properties and density of aluminum.
Chemical and physical properties
Without steel grade St 3, in our time it is impossible to build, construct above-ground and underground communications, produce vehicles, units and machine tools.
Impurities in steel of this grade are no more than:
- chromium - 0.30 percent;
- nickel - 0.30 percent;
- copper - 0.30 percent;
- sulfur - 0.005 percent;
- phosphorus - 0.04 percent;
- nitrogen - 0.10 percent.
Steel deoxidation
The steel deoxidation process is a chemical process that removes oxygen from the molten raw material. In this case, it is determined by an impurity that worsens the mechanical and physical properties of the alloy.
According to the deoxidation process, St3 steel is divided into the following types:
- Calm - deoxidation occurs with the use of manganese, silicon and aluminum.
- Boiling - deoxidation using only manganese.
- Semi-calm - deoxidation using aluminum and manganese.
The level of deoxidation is indicated in the marking of steel with the letters “kp”, “sp” and “ps”; their modification with an increased percentage of manganese is also indicated. For example – St3Gsp or St3Gps.
Boiling steel differs in chemical composition from calm steel in that the silicon content in it is very low, less than 0.05 percent. Calm steel contains more silicon, ranging from 0.16 to 0.30 percent. Since boiling steel contains more oxygen than calm steel, its quality is much worse than calm steel.
Semi-quiet steel occupies a middle position in quality between calm and boiling steels.
For the deoxidation process, elements such as silicon, manganese, and aluminum are used. The strength of their impact on steel varies. So, the “strongest” is aluminum, and the “weak” is manganese.
Quiet Steel is the most expensive steel in terms of cost. It lacks oxygen and is characterized by a homogeneous (homogeneous) structure, which, due to its nature, is designed to give the alloy maximum protection from environmental influences in the form of corrosion and ductility. Calm steel alloy St3 in accordance with GOST 380-2005 adopted in 2005, is used during the construction of rigid trusses and other metal structures, non-load-bearing and load-bearing elements. This grade of steel is used to make:
- sheet and packaged rolled products (steel sheet St 3);
- blanks for fittings and parts for pipelines (square pipe St 3);
- main and secondary elements for railway facilities, overhead and ground tracks, etc.
Semi-quiet steels occupy a neutral position between boiling and calm types of raw materials. In this form, a percentage of oxygen is already present, which gives the alloy less pronounced characteristics of ductility and hardness.
The chemical composition of this type of steel cannot be considered homogeneous. This grade of steel is used to produce rolled pipes and sheets, such a popular product as the St 3 beam. Semi-quiet steels are also used for the production of circles and strips, angles and squares, embedded parts and hexagons.
If we talk about boiling steels, then these are the most popular and affordable structural steel alloys. The production cost is low, but at the same time, blanks made from this steel (slabs, ingots, finished rolled sheets) lend themselves well to various processing under different thermal conditions.
The density of grade 3 steel of this modification is completely heterogeneous, however, subject to proper use and appropriate requirements, it is one of the most popular and inexpensive, practical types of alloys.
According to GOST 380-2005, it is stated that the manufacturer has the right to independently indicate the degree of deoxidation of raw materials if the customer has not determined it.
Mechanical performance
Mechanical indicators of the properties of St3 steel, which are used to control the properties of rolled raw materials:
- resistance that occurs temporarily - all categories;
- fluidity and its limit - all except the first category;
- bending under external influence in a cold state - all except the first category;
- relative lengthening - all existing categories are here;
- KCU (impact strength) at ambient temperature +20 °C – third category;
- KCU (impact strength) at an ambient temperature of –20 °C – fourth category;
- KCU (impact strength) after mechanical aging – fifth category;
- KC V (impact strength) at ambient temperature +20 °C – sixth category;
- KC V (impact strength) at an ambient temperature of –20 °C – seventh category.
Mechanical properties according to GOST 380-2005 standard
| Yield strength, σ0.2, MPa | Tensile strength, σв, MPa | Elongation at break, δ5, % |
| 205 — 255 | 370 — 490 | 23 — 26 |
Mechanical properties according to GOST 535-2005 standard
| Thickness, mm | Yield strength for residual deformation, σt, MPa | Tensile strength, σв, MPa | Elongation at break, δ5, % | Bend until the sides are parallel* |
| to 10 | > 255 | 380 — 490 | > 26 | d=a |
| 11 — 20 | > 245 | 370 — 480 | > 26 | d=a |
| 21 — 40 | > 235 | 370 — 480 | > 25 | d=2a |
| 41 — 100 | > 225 | 370 — 480 | > 23 | d=2a |
| more than 100 | > 205 | 370 — 480 | > 23 | d=2a |
By agreement with the consumer, for shaped steel with a thickness of more than 20 mm, the yield strength may be reduced by 10 MPa. The relative elongation may be reduced by 1% (by agreement with the consumer). The upper limit of tensile strength may be exceeded by 49 MPa, and by agreement with the consumer - without limiting the upper limit. limit of temporary resistance, provided that other standards are met. At the request of the consumer, exceeding the upper limit of temporary resistance is not allowed. * a - sample thickness, d - mandrel diameter Impact strength standards KCU, J/cm2
| Thickness, mm | At +20 °C | At -20°C | After mechanical aging |
| Steel category 3 (St3sp3)** | |||
| 3 — 9,9 | > 108 | — | — |
| 10 — 25 | > 98 | — | — |
| 26 — 40 | > 88 | — | — |
| Steel category 4 (St3sp4) | |||
| 3 — 9,9 | — | > 49 | — |
| 10 — 25 | — | > 29 | — |
| Steel category 5 (St3sp5) | |||
| 3 — 9,9 | — | > 49 | > 49 |
| 10 — 25 | — | > 29 | > 29 |
** Steel categories: 3 - steel with testing of mechanical properties for tensile strength on samples made from normalized blanks of the size specified in the order, but not more than 100 mm 4 - steel with testing of mechanical properties for tensile strength and impact strength on samples made from heat-treated (hardening + tempering) of workpieces of the size specified in the order, but not more than 100 mm 5 - steel with tensile testing of mechanical properties on samples made of steel in a cold-worked or heat-treated state (annealed or highly tempered)
Mechanical properties according to GOST 8696-74 standard
| Yield strength for residual deformation, σt, MPa (kgf/mm2) | Tensile strength, σв, MPa (kgf/mm2) | Elongation at break, δ5, % |
| > 245 (25) | > 372 (38) | > 23 |
Impact strength standards KCV, J/cm2 (kgf⋅m/cm2)
| Steel category* | At +20 °C | At -20°C | After mechanical aging |
| 3 (St3sp3) | > 59 (6) | ||
| 4 (St3sp4) | — | > 29,4 (3) | — |
| 5 (St3sp5) | — | > 29,4 (3) | > 29,4 (3) |
Mechanical properties according to GOST 10706-76 standard
| Assortment | Tensile strength, σв, MPa | Yield strength for residual deformation, σt, MPa | Elongation at break, δ5, % |
| Pipes | > 372 | > 245 | > 20 |
| Thickness, mm | Test temperature, °C | Impact strength KCU, J/cm2 |
| Steel category 3 (St3sp3)* | ||
| 5 — 9 | +20 | > 59 |
| 9 — 25 | +20 | > 49 |
| more than 25 | +20 | > 29 |
| Steel category 4 (St3sp4)* | ||
| 5 — 9 | -20 | > 20 |
| 9 — 25 | -20 | > 15 |
For main heating networks
| Tensile strength, σв, MPa | Yield strength for residual deformation, σt, MPa | Elongation at break, δ5, % | Test conditions | Impact strength KCU, J/cm2 |
| Steel category 4 (St3sp4)* | ||||
| > 372 | > 245 | > 23 | -20 °C | — |
| Steel category 5 (St3sp5)* | ||||
| > 372 | > 245 | > 23 | -20 °C | > 30 |
| > 372 | > 245 | > 23 | mechanical aging | > 29 |
* Steel categories depending on the standardized characteristics: 3 - steel with tensile mechanical properties tested on samples made from normalized workpieces of the size specified in the order, but not more than 100 mm 4 - steel with tensile and impact strength mechanical properties tested on samples, made from heat-treated (hardening + tempering) workpieces of the size specified in the order, but not more than 100 mm 5 - steel with tensile testing of mechanical properties on samples made from steels in a cold-worked or heat-treated state (annealed or highly tempered)
Mechanical properties according to GOST 10705-80 standard
| Assortment | Diameter, mm | Thickness, mm | Tensile strength, σв, MPa | Yield strength, σ0.2, MPa | Elongation at break, δ5, % |
| Heat-treated products | |||||
| Pipes | All | All | > 372 | > 225 | > 22 |
| Without heat treatment | |||||
| Pipes | from 10 to 19 | no more than 0.06*D | > 441 | > 216 | > 13 |
| Pipes | from 19 to 60 | no more than 0.06*D | > 392 | > 216 | > 13 |
| Pipes | from 10 to 19 | more than 0.06*D | > 441 | > 216 | > 5 |
| Pipes | from 19 to 60 | more than 0.06*D | > 392 | > 216 | > 5 |
| Pipes | from 60 to 152 | All | > 372 | > 216 | > 20 |
| Pipes | from 152 to 377 | no more than 6 | > 353 | > 216 | > 17 |
| Pipes | from 152 to 377 | more than 6 | > 353 | > 216 | > 14 |
| Pipes | more than 377 | no more than 6 | > 353 | > 216 | > 19 |
| Test temperature, °C | Impact strength KCU, J/cm2 (kgf⋅m/cm2) |
| +20 | 78,4 (8) |
| -20 | 39,2 (4) |
| +20 (after mechanical aging) | 39,2 (4) |
Mechanical properties according to GOST 5781-82 standard
| Assortment | Strength class | Diameter, mm | Tensile strength, σв, MPa** | Yield strength, σ0.2, MPa | Elongation at break, δ5, % | Cold bend test* |
| Armature | A-I (A240) | 6 — 40 | > 373 | > 235 | > 25 | 180 °C; c=d** |
* c - thickness of the mandrel, d - diameter of the rod ** For reinforcing steel with a diameter of 20 to 40 mm, cold bending test at 180 °C: c=2d
Weldability of steel grade St3
Consumers enjoy working with this grade of steel. Its technical characteristics, taking into account modifications, are very universal. One of the most important advantages of this brand is its excellent weldability.
Steel allows the use of automatic arc and manual welding methods, as well as contact-spot and electroslag methods. St. 3 is also used for the manufacture of forged parts (fences, various gratings, etc.).
Technological properties St3sp
This steel is considered universal in its qualities.
It is not subject to brittleness after tempering, does not form flakes, and is characterized by excellent weldability using any technology.
Definition and use of density
As you know, to find the density of a substance, its mass is divided by its volume. Density is a physical and chemical characteristic of a substance. She is constant. Materials for industrial production must meet this indicator. To denote it, it is customary to use the Greek letter ρ.
The density of iron is 7874 kg/m³, nickel - 8910 kg/m³, chromium - 7190 kg/m³, tungsten - 19250 kg/m³. Of course, this applies to hard alloys. In the molten state, substances have different characteristics.
In nature, only some metals are present in large quantities. The specific gravity of iron in the earth's crust is 4.6%, aluminum - 8.9%, magnesium - 2.1%, titanium - 0.63%. Metals are indispensable in most areas of human activity. Their production is growing year by year. For convenience, metals are divided into groups.
Iron and its alloys
Ferrous metals are usually called steel and cast iron of various grades. An alloy of iron and carbon is considered steel if the iron content is at least 45% and the carbon content is 0.1%-2.14%. Cast iron, accordingly, contains more carbon.
To obtain the necessary properties of steels and alloys, they are alloyed (alloying additives are added during remelting). This is how the specified grades are melted. All metal grades strictly comply with certain technical conditions. The properties of each brand are regulated by state standards.
Depending on the composition, the density of steel varies in the range of 7.6–8.8 (g/cm³) in the SGS or 7600–8800 (kg/m³) in the SI (this can be seen from Table 1). Of course, steel has a complex structure; it is not a mixture of different substances. However, the presence of these substances and their compounds changes properties, in particular density. Therefore, high-speed steels with a high tungsten content have the highest densities.
Non-ferrous metals and their alloys
Products made of bronze, brass, copper, aluminum are widely used in production:
- Bronzes are usually alloys of copper with tin, aluminum, lead and beryllium. However, in the Bronze Age, when the proportion of bronze in the total mass of metal products was almost 100%, these were copper-arsenic alloys.
- Zinc-based alloys - brass. Brass may contain tin, but its amount is less than zinc. Lead is sometimes added to produce free-flowing chips. In addition to jewelry alloys of brass and bronze, they are needed for machine and marine parts, hardware, and springs. Some varieties are used in aviation and rocketry.
- Duralumin (duralumin) - an alloy of aluminum and copper (copper 4.4%) is a high-strength alloy. Mainly used in aviation.
- Titanium is stronger than many steel grades. At the same time it is twice as light. These qualities have made it indispensable in most industries. It is also widely used in medicine (prosthetics). The share of titanium in the production of aircraft reaches 70% of all smelted in the world. About 15% of titanium is used for chemical engineering.
- Silver and gold are the first metals with which man became acquainted. Throughout the history of mankind, these metals have mostly been used for jewelry. And currently the trend continues.
- Due to its high refractoriness, tungsten is indispensable in instrument making. Its high density allows it to be used as radiation protection.
- Nickel and chromium form nichrome - a heat-resistant plastic alloy, very durable and reliable.
Different grades of steel and cast iron, bronze and other metals have different chemical compositions and different densities. The densities of all required materials are measured and systematized. Tables containing this data are available to users. With their help, you can easily find the mass of a product of a given shape.
General properties
Steel should not be confused with iron, which is a hard and relatively ductile metal with an atomic diameter of 2.48 angstroms, a melting point of 1535 °C and a boiling point of 2740 °C. Carbon, on the other hand, is a nonmetal with an atomic diameter of 1.54 angstroms, soft and brittle in most of its allotropes (diamond is the exception). The diffusion of this element in the crystal structure of iron is possible due to the difference in their atomic diameters. As a result of such diffusion, this material is formed.
The main difference between iron and steel is the percentage of carbon, which was indicated above. The material may have a different microstructure depending on a particular temperature. It can occur in the following structures (see the iron-carbon phase diagram for more information):
- perlite;
- cementite;
- ferrite;
- austenite.
The material retains the properties of iron in its pure state, but the addition of carbon and other elements, both metals and non-metals, improves its physical and chemical properties.
There are many types of steel depending on the elements added to it. The group of carbon steels consists of materials in which carbon is the only additive. Other specialty materials get their names from their basic functions and properties, which are determined by their structure and the additional elements added, such as silicon, cementitious, stainless, structural alloys and so on.
As a rule, all materials with additives are combined under one name - special steels, which differ from ordinary carbon steels, and the latter serve as the base material for the production of special materials. Such diversity of this material in its characteristics and properties has led to the fact that steel began to be called “an alloy of iron and another substance that increases its hardness.”
Metals and their density
Metallic materials are solids at room temperature and atmospheric pressure (the only exception is mercury). They have high ductility, electrical and thermal conductivity and have a characteristic shine when the surface is polished. Many properties of metals are associated with the presence of an ordered crystal lattice, in the nodes of which there are positive ionic cores connected to each other using a negative electron gas.
As for the density of metals, it varies within wide limits. Thus, the least dense are alkali light metals such as lithium, potassium or sodium. For example, the density of lithium is 534 kg/m3, which is almost two times less than the same value for water. This means that lithium, potassium and sodium plates will not sink in water. On the other hand, transition metals such as rhenium, osmium, iridium, platinum and gold have enormous densities, which are 20 or more times higher than the ρ of water.
Below is a table of metal densities. All values correspond to room temperature in g/cm3. If these values are multiplied by 1,000, we get ρ in kg/m3.
Why are there metals with high density and with low density? The fact is that the value of ρ for each specific case is determined by two main factors:
A feature of the metal crystal lattice. If this lattice contains atoms in the most dense packing, then its macroscopic density will be higher. FCC and HCP lattices have the densest packing. Physical properties of the metal atom. The greater its mass and the smaller the radius, the higher the value of ρ. This factor explains why high-density metals are chemical elements with a high number in the periodic table.
Metal components
The two main components of steel are found in abundance in nature, which favors its production on a large scale. The variety of properties and availability of this material makes it suitable for industries such as mechanical engineering, tool making, building construction, contributing to the industrialization of society.
Despite its density (the specific gravity of steel kg m3 is 7850, that is, the mass of steel with a volume of 1 m³ is equal to 7850 kilograms, for comparison, the density of aluminum is 2700 kg/m3) it is used in all sectors of industry, including aeronautics. The reasons for its such varied use are both its pliability and at the same time hardness, and its relatively low cost.
Additives and their characteristics
A special classification of steels determines the presence of a specific element in its composition and its percentage by weight. Elements are added to the alloy in order to give it specific properties, for example, increasing its mechanical endurance, hardness, wear resistance, melting ability, and others. Below is a list of the most common additives and the effects they cause.
- Aluminum : added in concentrations close to 1% to increase the hardness of the alloy, and at concentrations less than 0.008% as an antioxidant for heat-resistant materials.
- Boron : at low concentrations (0.001-0.006%) increases the hardenability of the material without reducing its ability to be machined. It is used in low quality materials, for example, in the production of plows and wire, ensuring its hardness and malleability. Also used as nitrogen traps in iron crystal structure.
- Cobalt . Reduces hardenability and leads to strengthening of the material and an increase in its hardness at high temperatures. Also increases magnetic properties. Used in heat-resistant materials.
- Chromium : due to the formation of carbides, it gives steel strength and resistance to high temperatures, increases corrosion resistance, increases the depth of formation of carbides and nitrides during thermochemical processing, is used as a hard stainless coating for axles, pistons, and so on.
- Molybdenum increases hardness and corrosion resistance for austenitic materials.
- Nitrogen is added to facilitate the formation of austenite.
- Nickel makes austenite stable at room temperature, increasing the hardness of the material. Used in heat-resistant alloys.
- Lead forms small globular formations that increase the machinability of steel. This element provides lubrication of the material at a percentage of 0.15% to 0.30%.
- Silicon increases the hardenability and oxidation resistance of the material.
- Titanium stabilizes the alloy at high temperatures and increases its resistance to oxidation.
- Tungsten forms stable and very hard carbides with iron that remain stable at high temperatures, 14-18% of this element creates a cutting steel that can be applied at three times the speed of conventional carbon steel.
- Vanadium increases the material's oxidation resistance and forms complex carbides with iron, which increase fatigue resistance.
- Niobium imparts hardness, ductility and malleability to the alloy. Used in structural materials and automation.
Impurities in the alloy
Impurities are elements that are undesirable in steel. They are contained in the material itself and enter it as a result of smelting, as they are contained in combustible fuels and minerals. It is necessary to reduce their content, since they deteriorate the properties of the alloy. In the case where their removal from the composition of the material is impossible or expensive, then they try to reduce their percentage to a minimum.
Sulfur: its content is limited to 0.04%. The element forms sulfides together with iron, which, in turn, together with austenite form a eutectic with a low melting point. Sulfides are released at grain boundaries. The sulfur content sharply limits the possibility of thermal and mechanical processing of materials at medium and high temperatures, since it leads to the destruction of the material along the grain boundaries.
Manganese additives allow you to control the sulfur content of materials. Manganese has a greater affinity with sulfur than iron, so instead of iron sulfide, manganese sulfide is formed, which has a high melting point and good plastic properties. The concentration of manganese must be five times greater than the concentration of sulfur to provide a positive effect. Manganese also increases the machinability of steels.
Phosphorus: the maximum limit for its content in the alloy is 0.04%. Phosphorus is harmful because it dissolves in ferrite, thereby reducing its ductility. Iron phosphide, together with austenite and cementite, forms a brittle eutectic with a relatively low melting point. The release of iron phosphide at grain boundaries makes the material brittle.
How to calculate P or perform 1 meter mass correction?
The actual method for determining density is very simple and is known to us from a school physics course. A sample of material is lowered into a measuring container filled with water to a specific mark. The water level rises to a specific height. The volume of displaced water is equal to the volume of the sample. The mass of the sample is formed by weighing on an accurate balance. Density will be equal to the ratio of mass and volume.
To correct the mass per linear meter or sq.m., you need to divide the value from the reference book by the density from the reference book and multiply the result by the measured density of the sample material. The corrected value will appear.
If similar calculations are expected to be repeated, then it will be more convenient to determine a correction indicator equal to the ratio of the typical density and the density of the sample, and then use it in calculations.
Density of metals
This is the most numerous group of the periodic table. A metal is any substance that has high thermal and electrical conductivity, a characteristic surface shine when polished, and the ability to undergo plastic deformation.
This chemical element has low electronegativity compared to substances such as nitrogen, oxygen and carbon. This fact leads to the fact that in bulk structures metal atoms form metallic bonds with each other. It represents the electrical interaction between positively charged ionic bases and a negative electron gas.
Metal atoms are arranged in space in an ordered structure called a crystal lattice. There are only three types:
- cubic;
- BCC (body-centered cubic);
- HPU (hexagonal close-packed);
- FCC (face centered cubic).
The density of metals is a physical quantity that depends on the type of crystal lattice. Below is a table of this parameter for all chemical elements in g/cm3, which under normal conditions are in a solid state.
It follows from the table that the density of metals is a value that varies over a wide range. Thus, the weakest is lithium, which, with the same volumes, is two times lighter than water. The density of the rare metal osmium is the highest in nature. It is 22.59 g/cm3.
Mechanical and technological characteristics of steel
It is very difficult to determine the specific physical and mechanical properties of steel, since the number of its types is varied due to different compositions and heat treatments, which allow the creation of materials with a wide variety of chemical and mechanical characteristics. This diversity has led to the fact that the production of these materials and their processing began to be separated into a separate branch of metallurgy - ferrous metallurgy, which differs from non-ferrous metallurgy. However, general properties for steel can be given; they are presented in the list below.
- The volumetric weight of steel, that is, the mass of 1 m³, is 7850 kg. The density of steel g cm3 is therefore 7.85.
- Depending on the temperature, the material can be bent, stretched and melted.
- The melting point depends on the type of alloy and the percentage of additives. Thus, pure iron melts at a temperature of 1510 °C, in turn, steel has a melting point equal to 1375 °C, which increases as the percentage of carbon and other elements in it increases (the exception is eutectics, which melt at lower temperatures). High-speed steel melts at a temperature of 1650 °C.
- The material boils at a temperature of 3000 °C.
- It is a deformation-resistant material whose hardness increases with the addition of other elements.
- It has relative malleability (it can be used to produce thin threads by drawing - wire), as well as ductility (you can produce flat metal sheets 0.12-0.50 mm thick - tin, which is usually coated with tin to prevent oxidation).
- Before using thermal treatment, the alloy undergoes mechanical processing.
- Some composites have shape memory and deform by an amount exceeding the yield strength.
- The hardness of steel varies between the hardness of iron and the hardness of structures that are obtained through thermal and chemical processes. Among them, the best known is hardening, which is applied to materials with a high carbon content. The high surface hardness of steel allows it to be used as a cutting tool. To obtain this characteristic, which is maintained at high temperatures, chromium, tungsten, molybdenum and vanadium are added to the steel. The hardness of metal is measured using Brinell, Vickers and Rockwell.
- Has good casting properties.
- The ability to corrode is one of the main disadvantages of steel, since oxidized iron expands in volume and leads to cracks on the surface, which in turn further accelerates the process of destruction. Traditionally, metal was protected from corrosion using various surface treatments. In addition, some steel compounds are resistant to oxidation, such as stainless steel materials.
- It has high electrical conductivity, which does not vary much depending on the composition of the alloy. In overhead power lines, aluminum conductors are most often used, which are covered with a steel jacket. The latter provides the necessary mechanical strength to the wires, and also contributes to their cheaper production.
- Used to produce artificial permanent magnets because magnetized steel does not lose its magnetic ability up to a certain temperature. In this case, the ferrite structure of steel has magnetic properties, while the austenite structure is not magnetic. To stabilize the ferrite structure, steel-based magnets usually contain about 10% nickel and chromium.
- With increasing temperature, a product made of this material increases its length. Therefore, if there are degrees of freedom in a particular structure, then thermal expansion is not a problem, but if such degrees of freedom do not exist, then the expansion of the steel will lead to additional stresses that must be taken into account. The coefficient of thermal expansion of steel is close to that of concrete. This fact makes it possible to use them together in structures of various types; this material is called reinforced concrete.
- It is a non-flammable material, but its fundamental mechanical properties quickly deteriorate when exposed to open flame.
Which is better: galvanized or stainless steel?
In order to successfully solve various technological problems and not get confused: buy a galvanized sheet instead of a stainless steel one, contact a trusted, reputable supplier. Although both metals are corrosion-resistant, and when constructing structures with a service life of no more than 10 years, it is quite possible to get by with cheaper galvanized steel, for critical objects it is still not worth skimping on quality.
A guarantee that you are purchasing a certified product will be choosing as a partner a reliable supplier with attractive market offers. Today we are the best and are ready to supply any volumes of highest quality metal products in the shortest possible time.
Areas of use
The process of extracting a valuable component from ore is carried out by crushing the rock, followed by enrichment of the material using the vibration-gravity method. In addition, a flotation method is used, which allows increasing the concentration of the material.
The concentrate is smelted in a furnace and reduced to a free state using charcoal. Chemically pure tin is extracted through the refining process using the electrochemical method.
Tin is used in various areas of production, in its pure form and as a component
Tin oxide, which is formed when the chemical element burns, is used as a polishing agent. The material is used to protect surfaces from corrosion by tinning.
Tin is used as an alloy component to make tin cans. It is part of solders. The strength and stability of tin-based compounds is given by the presence of copper and antimony in them. These properties are used in the manufacture of printing blocks and bearings for mechanisms.
The combination of a chemical element with sulfur is used to produce a golden-colored paint called gold leaf. Tin dioxide is used to prepare heat-resistant enamels and glazes.
Where and how is it used?
The performance qualities allow the alloy to be used for the manufacture of elements of welded structures operating under load, machine parts and mechanisms. The operating temperature must be above 0 degrees. The fifth category of rolled elements can be used in conditions of negative temperatures -40/-425 C under the action of a variable load.
Complex products require subsequent heat treatment; annealing is most often used. It reduces residual stresses after welding. The scope of application of ST3PS includes the production of At-400S fittings.
The sheets are used to produce parts using cold stamping. The result is pallets for collecting cutting fluids in production, containers of various volumes and purposes, casings, etc.
Method 4
Used to determine the density of liquids and gases in a small volume (1 – 2 ml) with an accuracy of ± 0.0001 g/cm3 using a density meter.
The principle of measuring density with a density meter is based on determining the period of oscillation of a U-shaped measuring tube of a certain volume caused by an electromagnetic generator.
The natural frequency of the tube depends on its design features (elasticity and mass) and is determined during the calibration process when it is filled with a substance of known density. When the tube is filled with the test substance, the vibration frequency of the tube changes depending on the mass (density) of the substance. The oscillation period of the measuring tube, measured by a special sensor, is automatically converted to the density of the sample in g/cm3.
Download in PDF OFS.1.2.1.0014.15 Density
Mechanical restoration
Parts made from steel grade ST3PS are processed using pre-selected equipment and speed. This ensures that the required performance indicators are maintained, reduces local stresses, etc.
Sharpening and milling is carried out using a cutting tool made of VK8 or T5K10. Internal and external threads are created using taps and dies made of R18 and R6M5 steel. When processing on a machine, it is necessary to use cutting fluids, and when processing manually, castor oil.
The impact strength of ST3PS steel allows products to be processed on machine tools under constant vibration load. The speed depends on the properties of the alloy; other parameters are also selected:
- thickness 6-10 cm – tool holder 16*25 mm;
- cutting depth 3 mm – feed speed 0.7-1.2 mm/rev;
- rotation speed 700 rpm.
Densities of some gases [edit | edit code ]
Density of gases, kg/m³ at low pressure.
| Nitrogen | 1,250 | Oxygen | 1,429 |
| Ammonia | 0,771 | Krypton | 3,743 |
| Argon | 1,784 | Xenon | 5,851 |
| Hydrogen | 0,090 | Methane | 0,717 |
| Water vapor (100 °C) | 0,598 | Neon | 0,900 |
| Air | 1,293 | Radon | 9,81 |
| Tungsten hexafluoride | 12,9 | Carbon dioxide | 1,977 |
| Helium | 0,178 | Chlorine | 3,164 |
| Dician | 2,38 | Ethylene | 1,260 |
To calculate the density of an arbitrary ideal gas under arbitrary conditions, you can use the formula derived from the equation of state of an ideal gas:
ρ = p MRT ho =>> ,
p is pressure, M is molar mass, R is the universal gas constant, equal to approximately 8.314 J/(mol K) T is thermodynamic temperature.
Application of steel St3
When considering different grades of steel, you need to take into account the fact that they are classified according to the degree of deoxidation. This chemical process involves removing oxygen from the composition. Too high an oxygen concentration determines a decrease in physical and mechanical properties.
The classification is carried out as follows:
- Calm is characterized by the fact that the composition includes from 0.16 to 0.3% silicon.
- Semi-calm has an average concentration of the element in question.
- Boiling differs in chemical composition from calm in that it contains at least 0.05% silicon.
St3 material is marked accordingly. Various substances can be used to carry out a chemical process.
It is worth considering that a quiet one is much more expensive than other options. This can be associated with the following points:
- The structure is homogeneous, thereby increasing the degree of protection of the material from environmental influences.
- The composition includes a small amount of oxygen, which determines high performance.
When using mild steel, the following products can be manufactured:
Rolled sheets and shapes. Fittings and parts that can be used to create a pipeline. Various pipes can be used to transport coolant or gas or other media. In order for them to withstand high loads and environmental influences, materials with strength and hardness must be used during manufacture.
In addition, attention is paid to cost, since alloys that are too expensive may be less practical to use. Steel 3 is more suitable for the manufacture of such products. Primary and secondary elements used in the manufacture of suspended structures and railway elements
In the railway industry, the metals that are most in demand are those that have low cost and high performance. Due to the large size of suspended structures, the price of one square meter is also of great importance.
Steel reinforcement
The semi-quiet variety of steel, which is also widely used, contains about one percent oxygen. Due to this, the characteristics of hardness and ductility are less pronounced. When using steel 3, the following can be produced:
- Pipes. This type of material is widely used today. Pipes are used to create a heating system as load-bearing elements. It is worth considering that pipes can have different diameters and wall thicknesses. The alloy in question has relatively low corrosion resistance, so it is necessary to protect the surface from exposure to high humidity.
- Sheet metal is also used extremely often, especially in the manufacture of cabinet products or cladding of load-bearing structures. The thickness can vary over a wide range. Rolled sheet metal can be used for cold bending or stamping. These two processes are characterized by high productivity. That is why the alloy in question is most widely used.
- Squares and corners are often used to obtain load-bearing structures. They are characterized by high strength, since the edges significantly increase rigidity and can distribute the load. Corners and squares are characterized by a large number of parameters: sheet thickness, angle of planes, length and cross-sectional shape. The scope of application is the manufacture of load-bearing structures and strengthening of existing structures.
- Various hexagons. They are also widely used and can be used in a wide variety of industries.
Steel sheet St3 hot rolled
Boiling alloys have become widespread due to their availability. They are the most affordable in terms of cost, and the resulting structure is characterized by a high degree of workability. In addition, the alloy lends itself well to heat treatment, but its performance is reduced due to the high oxygen concentration.
In conclusion, we note that many analogues of steel 3 have the appropriate performance characteristics. Foreign manufacturers use their own standards for labeling. At the same time, the concentration of harmful impurities is maintained within a certain range. The use of the most modern technologies makes it possible to reduce the amount of phosphorus and sulfur in the composition, making the material more durable and less fragile. In some cases, alloying elements are added.
Characteristics of steel ST3
All characteristics of steel st3 are regulated by GOST 380-71 standards. Its composition can include from 0.14 to 0.22% carbon. Steel 3 has quality properties, which are determined by weldability, mechanical properties and corrosion resistance. The mechanical characteristics determine which group the steel belongs to: high-strength, regular or high-strength.
Chemical composition of steel ST3
| steel grade | Mass fraction of elements, % | Carbon Manganese | Silicon |
| ST3kp | 0,14-0,22 | 0,30-0,60 | No more than 0.5 |
| ST3ps | 0,14-0,22 | 0,40-0,65 | 0,5-0,15 |
| ST3sp | 0,14-0,22 | 0,40-0,65 | 0,15-0,30 |
| ST3Gps | 0,14-0,22 | 0,80-1,10 | no more than 0.15 |
| ST3Gsp | 0,14-0,20 | 0,80-1,10 | 0,15-0,30 |
Impact strength of rolled steel ST3
| Steel grade* | Rolled thickness | Impact strength, J/cm2, not less | ||||
| KCU | KCV | |||||
| +20°С | -20°С | after mechanical aging | +20°С | 20°C | ||
| ST3ps ST3sp ST3Gps ST3Gsp | 3,0-5,0 | — | 49 | 49 | — | 9,8 |
| 5,1-10,0 | 108 | 49 | 49 | 34 | — | |
| 10,1-26,0 | 98 | 29 | 29 | 34 | — | |
| 26,1-40,0 | 88 | — | — | — | — | |
| * For ST3kp steel, impact strength is not standardized | ||||||
Mass fraction of steel elements St3sp according to GOST 380-2005
| C (Carbon) | Si (Silicon) | Mn (Manganese) | P (Phosphorus) | S (Sulphur) | Cr (Chrome) | Ni (Nickel) | Cu (Copper) | As (Arsenic) | Fe (Iron) |
| 0,14 — 0,22 | 0,15 — 0,3 | 0,4 — 0,65 | < 0,05 | < 0,05 | < 0,3 | < 0,3 | < 0,3 | < 0,08 | rest |
In open-hearth and converter smelting, the share of nitrogen is 0.01%. An increase in N < 0.013% is allowed, provided that the mass fraction of P is reduced by no less than 0.005% for each increase in the mass fraction of nitrogen by 0.001%. TU 14-1-5283-94: at the request of the consumer P < 0.035, S < 0.04.
Density of stainless steel
The density of a substance is calculated by dividing the mass of an object by its volume. Such calculations have already been made for all substances known to man, and metrological services periodically repeat and refine these measurements. In practice, people face another practical task: knowing the material from which the product is made, determine its mass.
The density of a substance is also called specific gravity (or, in everyday life, specific gravity) - that is, the mass of a solid physical body made of a given substance and having a unit volume.
Stainless steel
It should be noted that when using the term “mass”, in 99% of cases people are dealing with weight - the force of attraction of the physical body to the Earth. The fact is that to determine body weight in a strict physical sense, sophisticated equipment is required, available only in the largest scientific centers. For practical use, in most cases, conventional, more or less accurate scales using the Earth's gravity and springs, or levers and standard weights, or piezoelements are sufficient.
In practice, to calculate the weight of a linear or square meter of rolled metal, the specific gravity, or density of the material from which it is made, is used. In reference books on the assortment of rolled metal, among the main characteristics of each grade, the mass of a linear or square meter and the density value used in the calculations must be indicated.
However, you need to understand that the data in the directory is calculated based on the standard density of steel, most often it is 7.85 t/m3. At the same time, the actual density of a particular steel grade depends on the composition and specific amount of additives and can range from 7.6 to 8.8 t/m3.
This can give an error of up to 10% up or down for a product made from a very light or, conversely, very heavy alloy. For a small amount of metal the difference will be small and can be neglected. However, for complex products that use large volumes of metal, more accurate calculations will be required.
https://youtube.com/watch?v=eN9Y_AqExdI
The mass will be needed when creating an application for the purchase of metal. Based on the density of a given alloy, an adjustment is made to the reference values of the mass of one linear or square meter, and then the already specified value is used in the calculations.
Decoding
It is possible to determine what characteristics the ST3 steel material has in accordance with GOST thanks to decoding. According to GOST 380, this material is presented in the following varieties:
- Steel St3sp.
- Steel St3ps.
- Steel St3kp.
These indices are mandatory for any marking. When deciphering the brand of material, the following symbols must be taken into account:
- St - used to indicate the standard qualities of carbon steels.
- 3 — conditional number of the alloy grade. It can vary from 0 to 6, depending on the percentage of carbon in the material.
- G - this symbol is used if the material contains manganese. Thus, steel type St3gps is characterized by a content of 0.8% manganese.
- Sp - indicates the degree of deoxidation of steel. The abbreviation “kp” denotes boiling alloys, “ps” - semi-quiet alloys.
Thus, the St3ps5 grade is semi-calm, but is characterized by a high degree of deoxidation. The specifics of material marking are regulated by GOST 380-2005.
If the brand name does not contain the letters “ps” or “kp”, the steel should be considered calm.
Attention. The quiet variety ST3 is the most common, and therefore the letters “sp” can sometimes be omitted.. To understand the properties of products made from ST3 steel, you should focus on their GOSTs:
To understand the properties of products made from ST3 steel, you should focus on their GOSTs:
- GOST 107105-80 - for pipes and fittings for them;
- GOST 2591-2006 - for rental;
- GOST 14918-80 - for strip and strip rolling;
- GOST 5812-82 - for rails;
- GOST 8479-70 - for forgings.
Chemical composition
Decoding the steel grade St3 indicates the main components in its composition - iron (97%) and carbon (0.14-0.22%). The main quality of the alloy—its hardness—depends on the carbon concentration. The steel also contains small amounts of:
- manganese – 0.4-0.65%;
- silicon – 0.15-0.17%;
- nickel and chromium – 0.3% each;
- arsenic – 0.08%;
- copper – up to 0.3%;
- sulfur – 0.05%;
- phosphorus – 0.04%;
- nitrogen – up to 0.008%.
A feature of the St3 alloy is the strict regulation of the content of harmful impurities - sulfur and phosphorus. Phosphorus reduces the plasticity of the metal when exposed to high temperatures, and sulfur, when interacting with iron, forms sulfides, causing the phenomenon of red brittleness. It should be noted that there is an increased concentration of nitrogen, which accounts for almost 0.1%. In accordance with GOST 380-2005, the alloy is marked with accompanying indices that indicate the degree of deoxidation, for example, St3Gsp:
- the first two letters indicate carbon steel of ordinary quality;
- the number “3” means the serial number of the brand according to this GOST;
- the sign “G” indicates a modification with a high manganese content;
- “sp”, “kp”, “ps” – degrees of deoxidation.
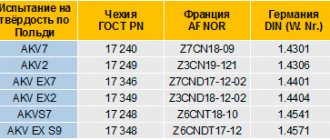
Substitutes for steel grade St3 can be:
- C245, according to GOST 27772-88;
- C285;
- VSt3Sp.
Foreign analogues are labeled according to different rules:
- A57036, K01804 – USA;
- 40B, 722M24, HFS4 – UK;
- 1.0038, DC03 – Germany;
- E24-2, E24-4 – France;
- SS330, SS400 – Japan;
- Fe360B, Fe360C – Italy;
- G235C – China;
- RSt360B – Austria;
- Fe235D – Hungary.
The product range includes:
- long and shaped steel according to GOST 2591-2006;
- sheets of various thicknesses and stampings;
- pipes and fittings, according to GOST 10705-80;
- tapes and strips that are produced in accordance with GOST 14918-80;
- wire of different sections.
Determination of product mass
All modern reference materials, GOST and technical specifications of enterprises have been adjusted in accordance with the international classification.
Using reference tables of densities of various materials, it is easy to determine their mass. This is especially true when items are heavy or appropriate scales are not available. To do this, you need to know their geometric parameters. Most often, you need to find out the mass of an object in the form of a cylinder, pipe or parallelepiped:
- Metal rods are cylindrical in shape. Knowing the diameter and length, it is easy to find out the mass. Mass equals density times volume. Finding the volume of an object. It is obtained by multiplying the cross-sectional area by the length. The area of a circle, knowing the diameter, is easy to determine. The squared diameter is multiplied by 3.14 (pi), divided by 4.
- We obtain the mass of the pipe in the same way. When finding the area, we take the difference between the outer and inner diameter of the section.
- To determine the mass of a sheet, bloom, slab or bar of rectangular cross-section, we determine the volume by multiplying the length, height and thickness. Multiply by the density from the reference book.
https://youtube.com/watch?v=jW8agjKkd4I
With such calculations, a small error is always allowed, because the shapes are not ideal. In practice it can be neglected. Manufacturers of metal products have developed special mass calculators for users. It is enough to enter unique dimensions in the appropriate windows and get the result.
Analogs
As already noted, the St3 grade is in demand in the production of various structures, and in fact, is the most popular structural steel. This is the reason why it is produced by metallurgical plants located in all parts of the world, for example:
- USA - A284Gr.D, A57036;
- Germany - 1.0038;
- Japan - SS330;
- European Union - Fe37-3FN;
- China - Q235.
Suppliers of steel produced outside our country must submit documents confirming the compliance of imported materials with domestic GOST and TU.
Similar foreign steels
The metal is one of the most common structural steels. Therefore, analogues of ST3PS include foreign products:
- Q235;
- S235J0;
- Fe235D;
- 1.0038;
- K01804 and D.
When purchasing foreign product ranges, you must ensure that they comply with GOST and TU requirements.
Sources
- https://nzmetallspb.ru/stanki/stal-st3-marki-harakteristiki-himicheskij-sostav.html
- https://molotok34.ru/spravochnik/st3ps-rasshifrovka-stali.html
- https://svarkaipayka.ru/material/stal/tehnicheskie-harakteristiki-uglerodistoy-stali-3.html
- https://promkrepez.ru/info/steel-grades/stal-marki-st3/
- https://metal.place/ru/wiki/st3sp/
- https://punktpriema.ru/articles/tehnicheskie-harakteristiki-konstruktsionnoy-stali-st3.html
- https://stanok.guru/stal/fiziko-himicheskie-harakteristiki-staley-st3.html
General information about products
The average density of steel is 8.0 g/cm³. Density depends on how much carbon is contained and what alloying substances were used. The average density of stainless steel is 7.9 g/cm³.
Almost all alloys discussed in this article are made for structural material, in the broad sense of the word. The main qualities required for this material are strength and ductility. It is necessary that the material can withstand sufficient loads during operation and not break.
There are more than 1,500 grades, each intended for a specific product, so the specific gravity of steel varies greatly between grades.
For example, in order to produce bearings, special chromium ball bearing steel grades ShKh15 and ShKh15SG are required. It is characterized by high hardness, strength and contact endurance. The density of stainless steel of this brand is 7.65 g/cm³.
Various elastic elements, such as leaf springs, must be made of a material that can withstand high deformation of the part.
Brands 50HFA, 30Х13, 03Х12Н10Д2Т have such important characteristics as high elasticity, endurance, and fluidity. The density of steel of these grades is 7.6 g/cm³
40ХН2МА is a grade of alloy containing a medium amount of carbon and alloying elements, so it has high strength and is quite ductile. The density of this grade of steel is 7.8 g/cm³. High-strength structures are made from it.
Since time immemorial, humanity has been mining iron and making various tools from it. Currently, steel production is the leading industry. Almost everything that surrounds us contains steel: cars, building frames, household items, tools, etc.
Density of carbon steels
The density of carbon steel at room temperature ranges from 7.83 to 7.87 g/cm 3 . The table shows the density values of the following carbon steels: steel 08KP, steel 08, steel 20, steel 40, steel U8, steel U12.
The density values in the table are indicated depending on the temperature - in the range from 0 to 1100°C. When steel is heated, it becomes less dense. For example, the density of steel 20 is 7859 kg/m 3 at a temperature of 15°C, and when heated to a temperature of 1100°C, the density of this steel decreases to 7496 kg/m 3 .
Note: The density of carbon steels in the table is expressed in units of kg/m3.
PROPERTIES OF LIQUID STEEL
Liquid steel is an alloy of iron with various impurity elements. The combinations of these impurities are diverse, so the properties of liquid steel vary over a wide range. If we determine with high accuracy the dependence of the properties of liquid steel on changes in temperature or concentration of impurities, then a non-monotonic (jump-like) change in properties is characteristic of changes in the structure of the liquid (disorder or, conversely, association of atoms, the appearance of microgroups, delamination, etc.). Properties of a liquid that depend on changes in its structure are called structure-sensitive.
These primarily include density, viscosity, surface tension, electrical conductivity, thermal conductivity, speed of sound, etc. In steel metallurgy, data on density, viscosity and surface tension are most often used.
Density is one of the most important structure-sensitive properties and is determined by the expression sp, where V
sp—specific volume of liquid (or solid) metal;
V
beat =
Vat + V
St
,
where
Vat
is the sum of the volume of atoms or molecules, which does not change with changes in temperature and pressure;
V
St is the free space between atoms (molecules), which changes when external conditions change.
When Vst
, the density will also change. If this change has an abrupt nature, then under given conditions (temperature, impurity concentration, etc.) there is a change in the structure (structure) of liquid steel.
Thus, kinks or fractures observed in density polytherms (curves of changes in melt temperature) or isotherms (curves of changes in melt composition at a given temperature) indicate certain changes in the structure of the melt. Most studies have noted a linear (without kinks) nature of the change in the density of liquid metals with temperature, however, in some studies, kinks have been found in density polytherms.
There is a relationship between the type of metal crystal lattice and the change in density during melting. Metals with dense crystal lattices melt with an increase in volume, a decrease in density and coordination number. Metals having “loose” crystal lattices (tetragonal, rhombohedral and
etc.), melt with increasing density and coordination number and decreasing specific volume. Such metals include, for example, bismuth, antimony, etc. Iron has a dense lattice. The density of iron at 1600 °C is ~7.0 g/cm3; with further increase in temperature it decreases.
Viscosity, like density, is the most important physical and chemical property of a liquid. Viscosity (internal friction) characterizes the property of fluid bodies (liquids and gases) to resist the irreversible movement of one part relative to another during shear, stretching or other types of deformation. The fundamental law of viscous flow was established by Newton:
S
where F—
tangential (tangential) force causing a shift of layers of liquid (gas) one relative to another;
— proportionality coefficient, called the coefficient of dynamic viscosity
or
viscosity,
Pa • s (the same as N • s/m2).
The reciprocal of viscosity (1/n) is called fluidity;
ratio (v2 - v1)/(z2 –z1
\) -
flow velocity gradient (rate of change from layer to layer), or shear rate; S is the area of the layer along which the shift occurs.
Along with dynamic viscosity, the value v = /р (р is the density of the liquid), called kinematic viscosity
(m2/s or cm2/s).
Instruments used to determine the viscosity of liquids (and gases) are called viscometers,
and the branch of physics devoted to measuring viscosity is called
viscometry
(see Section 9.3).
The viscosity of water at 25 ºС is 0.00089 Pa-s, glycerol -0.5 Pa • s. The viscosity at 1600 °C of pure iron, according to various sources, is 0.0045-0.0060 Pa • s, the viscosity of steel, depending on its composition, is 0.005-0.0085 Pa • s, open-hearth slag - 0.02-0, 04 Pa • s.
In liquids, viscosity is the result primarily of intermolecular interactions that limit the mobility of molecules. A molecule from one layer can penetrate into an adjacent layer only if there is a cavity in it sufficient for the molecule to slip into it. The formation of a cavity (“loosening” of the liquid) is associated with energy consumption. This so-called activation energy of viscous flow
decreases with increasing temperature and decreasing pressure.
In 1912 Russian physicist L.I. Bachinsky, based on the assumption that the viscous properties of a liquid are determined by the forces of intermolecular interaction, established the relationship between the coefficient of dynamic viscosity and specific volume V:
c/(Vb)
where with
and
b are
constants.
Constant b
close to the specific volume of the solid at the moment of melting V
;
accordingly, the difference
V - b
represents the so-called
free volume of liquid.
The larger this free volume, the lower its viscosity.
In the Baczynski formula, the effect of temperature on viscosity is taken into account through the specific volume of the liquid V,
since it directly depends on temperature. As the temperature increases, the viscosity decreases, since this causes the liquid to loosen (which consumes energy).
Taking into account the difference in volumes of liquid and solid metals Vl-
We get Vtv =
s/(
Vl - Vtv). The difference Vl - Vtv characterizes the degree of loosening of the liquid, or the total volume of vacancies.
Ya. I. Frenkel, when developing the kinetic theory of liquids, proposed using a formula characterizing the relationship between viscosity and temperature:
=Aexp(E /RT). ln =lnA+E /RT
where E
is
the activation energy of viscous flow, characterizing the energy required for the transition of a particle (or group of particles) from one equilibrium position to another.
In accordance with this formula, the quantity is a function of \/T,
therefore the dependence of viscosity on temperature is usually expressed graphically in the coordinates
ln -I/T.
In the case of a change in the structure of the liquid metal at temperatures corresponding to a change in the structure (structure) of the liquid metal, a fracture is observed in the graph of this function. When considering experimental data on the toughness of steel, it is necessary to remember that impurities, especially non-metallic inclusions, significantly increase the toughness. The influence of impurities in liquid iron manifests itself in increased interparticle interaction and a decrease in the mobility of iron atoms, leading to an increase in viscosity. In addition to impurities, the viscosity of steel is noticeably influenced by other factors (non-metallic inclusions, gases, etc.).
Viscosity hysteresis. Numerous experiments are known in which the hysteresis of the viscosity of liquid steel was established, which consists in the discrepancy between the viscosity values obtained in the heating and cooling modes of the metal: the viscosity of the melt in the cooling mode after heating is often higher than the viscosity during initial heating. Hysteresis is especially noticeable for alloy steels. When explaining this phenomenon, the term “heterogeneity of the structure of liquid steel” is sometimes used. This usually implies the phenomenon of preservation or creation of slowly disintegrating groups or lattices, distinguished by the presence of certain connections. The composition and size of these groups depend on the composition of the steel and smelting technology. It is assumed that for each steel there is a certain critical temperature, upon reaching which a quasi-homogeneous melt structure is formed, eliminating viscosity hysteresis.
There is a connection between the properties of steel and its viscosity in the liquid state. Simultaneously with obtaining a quasi-homogeneous structure of the liquid, as a result of eliminating viscosity hysteresis, maximum plasticity and impact strength of steel are achieved
in a solid state; At the same time, the strength properties of steel decrease.
A series of studies of the properties of liquid steel was carried out by the Ural scientists P.V. Geld, B.A. Baum and others. The results of these studies indicate that most steels and alloys are characterized by differences in viscosity and electrical resistivity during heating and cooling. Researchers on this issue suggest that the hysteresis of viscosity and electrical resistance is explained by changes in the structure of the melts.
The most common (according to these scientists) three forms of viscosity hysteresis are shown in Fig. 10.2. The case when hysteresis appears only at a certain overheating above the liquidus line (tr-
temperature of the beginning of branching of polytherms or the beginning of hysteresis), is shown in Fig.
10.2, a.
With greater overheating, the position of the polytherms does not change.
According to Held and Baum, who proposed this theory, in this case, apparently, changes in the nonequilibrium structure and the approach of the melt to the equilibrium state, starting from a certain temperature, occur monotonically and end at tr.
In Fig.
10.2, b shows the case when hysteresis is observed only when the melt is heated to temperatures exceeding the temperature of the anomalous decrease in properties /an. At this temperature, an abrupt change in the structure of the melt occurs, which causes an anomalous increase in viscosity and a rapid transition to an equilibrium state. Finally, in Fig. 10.2c illustrates
the case when hysteresis is observed only when heated to
a critical temperature t
cr, heating to which upon subsequent cooling causes branching of the polytherms.
According to B. A. Baum and G. V. Tyagunov, one of the possible explanations for this dependence is as follows. The melt has at least two structural components, for example, carbide-like complexes and a metal matrix. When heated, the energy of thermal motion of particles increases in proportion to the absolute temperature, and the stability of interatomic bonds decreases non-monotonically. However, this non-monotonicity during heating may not manifest itself in this property if changes in individual structural components are interrelated and compensate for one another. They are completely completed only near t
cr.
During the reverse decrease in temperature, the disappeared nonequilibrium structure is not restored, but the forces of interatomic interaction still manifest themselves non-monotonically. Thus, in the mentioned model, carbon atoms again become neighbors of atoms of carbide-forming elements. This worsens the conditions for their mutual movement and is manifested in a sharp increase in viscosity at tr.
All of the above is only one possible explanation for the observed factors. At present, there is no convincing interpretation of the observed phenomena of viscosity hysteresis. Other discovered phenomena are also unclear: for example, in many (but not all) cases, hysteresis is observed only during the primary heating and cooling cycle; for some alloy steels (for example, ball bearing steels), remelting does not change the hysteresis; for many groups
Rice. 10.2. Forms of viscosity hysteresis 108
alloy steels, the lower the plasticity of solid samples, the greater the hysteresis.
Liquid steel is an alloy of iron with various impurity elements. The combinations of these impurities are diverse, so the properties of liquid steel vary over a wide range. If we determine with high accuracy the dependence of the properties of liquid steel on changes in temperature or concentration of impurities, then a non-monotonic (jump-like) change in properties is characteristic of changes in the structure of the liquid (disorder or, conversely, association of atoms, the appearance of microgroups, delamination, etc.). Properties of a liquid that depend on changes in its structure are called structure-sensitive.
These primarily include density, viscosity, surface tension, electrical conductivity, thermal conductivity, speed of sound, etc. In steel metallurgy, data on density, viscosity and surface tension are most often used.
Density is one of the most important structure-sensitive properties and is determined by the expression sp, where V
sp—specific volume of liquid (or solid) metal;
V
beat =
Vat + V
St
,
where
Vat
is the sum of the volume of atoms or molecules, which does not change with changes in temperature and pressure;
V
St is the free space between atoms (molecules), which changes when external conditions change.
When Vst
, the density will also change. If this change has an abrupt nature, then under given conditions (temperature, impurity concentration, etc.) there is a change in the structure (structure) of liquid steel.
Thus, kinks or fractures observed in density polytherms (curves of changes in melt temperature) or isotherms (curves of changes in melt composition at a given temperature) indicate certain changes in the structure of the melt. Most studies have noted a linear (without kinks) nature of the change in the density of liquid metals with temperature, however, in some studies, kinks have been found in density polytherms.
There is a relationship between the type of metal crystal lattice and the change in density during melting. Metals with dense crystal lattices melt with an increase in volume, a decrease in density and coordination number. Metals having “loose” crystal lattices (tetragonal, rhombohedral and
etc.), melt with increasing density and coordination number and decreasing specific volume. Such metals include, for example, bismuth, antimony, etc. Iron has a dense lattice. The density of iron at 1600 °C is ~7.0 g/cm3; with further increase in temperature it decreases.
Viscosity, like density, is the most important physical and chemical property of a liquid. Viscosity (internal friction) characterizes the property of fluid bodies (liquids and gases) to resist the irreversible movement of one part relative to another during shear, stretching or other types of deformation. The fundamental law of viscous flow was established by Newton:
S
where F—
tangential (tangential) force causing a shift of layers of liquid (gas) one relative to another;
— proportionality coefficient, called the coefficient of dynamic viscosity
or
viscosity,
Pa • s (the same as N • s/m2).
The reciprocal of viscosity (1/n) is called fluidity;
ratio (v2 - v1)/(z2 –z1
\) -
flow velocity gradient (rate of change from layer to layer), or shear rate; S is the area of the layer along which the shift occurs.
Along with dynamic viscosity, the value v = /р (р is the density of the liquid), called kinematic viscosity
(m2/s or cm2/s).
Instruments used to determine the viscosity of liquids (and gases) are called viscometers,
and the branch of physics devoted to measuring viscosity is called
viscometry
(see Section 9.3).
The viscosity of water at 25 ºС is 0.00089 Pa-s, glycerol -0.5 Pa • s. The viscosity at 1600 °C of pure iron, according to various sources, is 0.0045-0.0060 Pa • s, the viscosity of steel, depending on its composition, is 0.005-0.0085 Pa • s, open-hearth slag - 0.02-0, 04 Pa • s.
In liquids, viscosity is the result primarily of intermolecular interactions that limit the mobility of molecules. A molecule from one layer can penetrate into an adjacent layer only if there is a cavity in it sufficient for the molecule to slip into it. The formation of a cavity (“loosening” of the liquid) is associated with energy consumption. This so-called activation energy of viscous flow
decreases with increasing temperature and decreasing pressure.
In 1912 Russian physicist L.I. Bachinsky, based on the assumption that the viscous properties of a liquid are determined by the forces of intermolecular interaction, established the relationship between the coefficient of dynamic viscosity and the specific volume V:
c/(Vb)
where with
and
b are
constants.
Constant b
close to the specific volume of the solid at the moment of melting V
;
accordingly, the difference
V - b
represents the so-called
free volume of liquid.
The larger this free volume, the lower its viscosity.
In the Baczynski formula, the effect of temperature on viscosity is taken into account through the specific volume of the liquid V,
since it directly depends on temperature. As the temperature increases, the viscosity decreases, since this causes the liquid to loosen (which consumes energy).
Taking into account the difference in volumes of liquid and solid metals Vl-
We get Vtv =
s/(
Vl - Vtv). The difference Vl - Vtv characterizes the degree of loosening of the liquid, or the total volume of vacancies.
Ya. I. Frenkel, when developing the kinetic theory of liquids, proposed using a formula characterizing the relationship between viscosity and temperature:
=Aexp(E /RT). ln =lnA+E /RT
where E
is
the activation energy of viscous flow, characterizing the energy required for the transition of a particle (or group of particles) from one equilibrium position to another.
In accordance with this formula, the quantity is a function of \/T,
therefore the dependence of viscosity on temperature is usually expressed graphically in the coordinates
ln -I/T.
In the case of a change in the structure of the liquid metal at temperatures corresponding to a change in the structure (structure) of the liquid metal, a fracture is observed in the graph of this function. When considering experimental data on the toughness of steel, it is necessary to remember that impurities, especially non-metallic inclusions, significantly increase the toughness. The influence of impurities in liquid iron manifests itself in increased interparticle interaction and a decrease in the mobility of iron atoms, leading to an increase in viscosity. In addition to impurities, the viscosity of steel is noticeably influenced by other factors (non-metallic inclusions, gases, etc.).
Viscosity hysteresis. Numerous experiments are known in which the hysteresis of the viscosity of liquid steel was established, which consists in the discrepancy between the viscosity values obtained in the heating and cooling modes of the metal: the viscosity of the melt in the cooling mode after heating is often higher than the viscosity during initial heating. Hysteresis is especially noticeable for alloy steels. When explaining this phenomenon, the term “heterogeneity of the structure of liquid steel” is sometimes used. This usually implies the phenomenon of preservation or creation of slowly disintegrating groups or lattices, distinguished by the presence of certain connections. The composition and size of these groups depend on the composition of the steel and smelting technology. It is assumed that for each steel there is a certain critical temperature, upon reaching which a quasi-homogeneous melt structure is formed, eliminating viscosity hysteresis.
There is a connection between the properties of steel and its viscosity in the liquid state. Simultaneously with obtaining a quasi-homogeneous structure of the liquid, as a result of eliminating viscosity hysteresis, maximum plasticity and impact strength of steel are achieved
in a solid state; At the same time, the strength properties of steel decrease.
A series of studies of the properties of liquid steel was carried out by the Ural scientists P.V. Geld, B.A. Baum and others. The results of these studies indicate that most steels and alloys are characterized by differences in viscosity and electrical resistivity during heating and cooling. Researchers on this issue suggest that the hysteresis of viscosity and electrical resistance is explained by changes in the structure of the melts.
The most common (according to these scientists) three forms of viscosity hysteresis are shown in Fig. 10.2. The case when hysteresis appears only at a certain overheating above the liquidus line (tr-
temperature of the beginning of branching of polytherms or the beginning of hysteresis), is shown in Fig.
10.2, a.
With greater overheating, the position of the polytherms does not change.
According to Held and Baum, who proposed this theory, in this case, apparently, changes in the nonequilibrium structure and the approach of the melt to the equilibrium state, starting from a certain temperature, occur monotonically and end at tr.
In Fig.
10.2, b shows the case when hysteresis is observed only when the melt is heated to temperatures exceeding the temperature of the anomalous decrease in properties /an. At this temperature, an abrupt change in the structure of the melt occurs, which causes an anomalous increase in viscosity and a rapid transition to an equilibrium state. Finally, in Fig. 10.2c illustrates
the case when hysteresis is observed only when heated to
a critical temperature t
cr, heating to which upon subsequent cooling causes branching of the polytherms.
According to B. A. Baum and G. V. Tyagunov, one of the possible explanations for this dependence is as follows. The melt has at least two structural components, for example, carbide-like complexes and a metal matrix. When heated, the energy of thermal motion of particles increases in proportion to the absolute temperature, and the stability of interatomic bonds decreases non-monotonically. However, this non-monotonicity during heating may not manifest itself in this property if changes in individual structural components are interrelated and compensate for one another. They are completely completed only near t
cr.
During the reverse decrease in temperature, the disappeared nonequilibrium structure is not restored, but the forces of interatomic interaction still manifest themselves non-monotonically. Thus, in the mentioned model, carbon atoms again become neighbors of atoms of carbide-forming elements. This worsens the conditions for their mutual movement and is manifested in a sharp increase in viscosity at tr.
All of the above is only one possible explanation for the observed factors. At present, there is no convincing interpretation of the observed phenomena of viscosity hysteresis. Other discovered phenomena are also unclear: for example, in many (but not all) cases, hysteresis is observed only during the primary heating and cooling cycle; for some alloy steels (for example, ball bearing steels), remelting does not change the hysteresis; for many groups
Rice. 10.2. Forms of viscosity hysteresis 108
alloy steels, the lower the plasticity of solid samples, the greater the hysteresis.
Definition and characteristics of density
Density is a physical quantity that determines the ratio of mass to volume. Almost all materials are characterized by a similar physical and mechanical indicator. It is worth considering that the corresponding density of aluminum, copper and cast iron differ significantly.
The considered physical and mechanical quality determines:
- Some physical and mechanical properties. In most cases, an increase in density is associated with a decrease in the grain structure. The smaller the distance between individual particles, the stronger the bond formed between them, the hardness increases and the ductility decreases.
- As the distance between particles decreases, their number and weight of the material increase. Therefore, when creating cars, airplanes and other equipment, a material is selected that is lightweight and sufficiently durable. For example, the density of aluminum kg m3 is about 2,700, while the density of metal kg m3 is more than twice that.
There are special tables of metal density, which indicate the indicator in question for steel and non-ferrous alloys, as well as cast iron.
Read also: Data sheet for Mercury electricity meter