The industry uses natural graphite, as well as artificial varieties of this material. Its wide range of applications is due to its unique physical and chemical properties, which may differ slightly for different brands and types of graphite.
IMPORTANT PHYSICAL AND CHEMICAL INDICATORS OF GRAPHITE:
- Thermal conductivity;
- Electrical conductivity;
- Expansion due to heat;
- Strength;
- Solubility;
- Wettability;
- Anisotropy of properties.
THERMAL CONDUCTIVITY OF GRAPHITE:
- Natural types of this material are characterized by high thermal conductivity (many metals are inferior to graphite in this indicator). It is influenced by the final processing temperature of a particular grade of graphite, but the average is 3.55 W*degrees/cm, and the thermal conductivity coefficient is 0.041. It should be noted that thin graphite filaments conduct heat better than copper counterparts.
- At a pressure of 0.9-1 atmosphere, graphite boils, reaching a temperature of 4200 degrees, and melts at 3845-3890 degrees.
- Crystalline varieties of the material ignite in an oxygen stream at 700-730 degrees Celsius.
- Combustion of graphite releases a sufficient amount of heat - up to 7856 kcal.
Basic properties
If you are interested in the question of what is the density of graphite, you should know that this parameter is 2230 kg/m3. Another allotrope of carbon is diamond, which is why graphite is sometimes compared to it. The latter has electrically conductive characteristics and acts as a semimetal. This property has found its application in the electrode production process.
The density of graphite is not all you need to know if you are interested in this mineral. It is necessary to take an interest in other properties as well. For example, this crystalline modification of carbon does not melt, but ignites when exposed to a temperature of 3500 °C. The material passes through the liquid phase, turning into a gaseous state.
However, if the conditions include increasing the pressure to 90 MPa, as well as the temperature, then melting can be achieved. This discovery was made while studying the properties of diamond when they tried to synthesize it. But it was not possible to obtain this material from molten graphite.
Therapeutic effect
Homeopaths were the first to appreciate graphite. They found that the mineral is suitable for the treatment of skin pathologies (eczema, psoriasis, lichen, etc.).
Today the list has been expanded:
- Metabolic disease.
- Malfunction of the thyroid gland.
- Respiratory tract diseases (rhinitis, bronchial asthma).
- Gastrointestinal problems (gastritis, gastric ulcer, duodenal ulcer, colitis).
- Women's ailments (amenorrhea, chronic inflammation of the ovaries, mastopathy).
- Conjunctivitis, cataracts, stye.
The mineral also “supervises” emotional health. It is prescribed for morning headaches, neurasthenia, apathy, and depression.
Crystal cell
The crystal lattice of graphite provides for the presence of carbon atoms. It has a layered structure. The distance between individual layers can reach 0.335 nm. In a lattice, carbon atoms bond with three other carbon atoms.
The lattice can be hexagonal or rhombohedral. In each layer, the carbon atoms are located opposite the central parts of the hexagons. The latter are located in adjacent layers, then the position of the layers is repeated, which happens after one.
Where and how is it mined?
There are commercial-scale graphite deposits on all continents:
- Both Americas - USA, Canada, Brazil;
- Europe – Germany, Greenland, Italy;
- Australia.
The raw materials of each graphite mine can be distinguished by structure, color, and other characteristics.
Russia has three largest deposits:
- Buryatia – high-quality densely crystalline raw materials.
- Krasnodar region (two) – dense, finely crystalline, scaly, graphite shales.
Graphites are formed by coal pyrolysis or under the influence of extremely high temperatures and pressure. For example, the outpouring of magma onto coal deposits.
It is mined by above-ground or underground methods. Graphite crystals are found in shales, marbles, and other organic rocks.
The annual global production of graphite is 600 thousand tons.
Production of artificial graphite
Graphite and its properties are not the only thing you should know if you are interested in this mineral. It is also important to inquire about the production of an artificial variety. It differs from natural material in that during synthesis a substance with specified parameters is obtained.
Waste petroleum coke and coal sand are used for production. The mixture of fine-grained elements is fired and then cooled for about 5 weeks. The impact of temperature at the first stage is accompanied by its increase to 1200 °C.
To increase the theoretical density of graphite, the workpieces are impregnated with sand. At the final stage, graphitization occurs; it involves heat treatment of the material in a special furnace, where the temperature reaches 3000 °C. In this case, it is possible to form a crystal lattice.
This graphite has high thermal conductivity and excellent electrical conductivity. Anisotropy of properties is inherent in a mineral obtained by extrusion. Today, a newer technology is used, which is called isostatic pressing. This makes it possible to produce a material with a low coefficient of friction. It has isotropic properties.
The density of graphite (g/cm3), which is obtained during the extrusion process, reaches 2.23. The same indicator for the isostatic recrystallized variety, depending on the brand, can reach 5 g/cm3. This material is used for the production of large-sized workpieces, the length and diameter of which are 1000 and 500 mm, respectively, as well as for the production of casting parts and molds that have anti-friction properties.
Physico-chemical characteristics
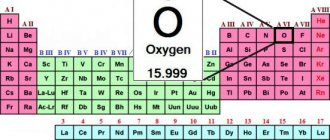
According to chemical nomenclature, the mineral graphite is pure carbon with a formula of one symbol (C).
The composition is sometimes supplemented by absorbed gas, bitumen, water, and mechanical impurities.
| Formula | C (carbon) |
| Color | Grey, black steel |
| Stroke color | Black |
| Shine | Metallic |
| Transparency | Opaque |
| Hardness | 1–2 |
| Cleavage | Perfect by {0001} |
| Density | 2.09–2.23 g/cm³ |
| singonia | Hexagonal (planaxial) |
The mineral class according to international nomenclature is native element. According to the taxonomy of the USSR, it is a non-metal, but is endowed with characteristics inherent in metals - electrical conductivity, magnetism.
Main brands
Today, the possibility of synthesis with different grain sizes is used. As a result, graphite can be classified into:
- coarse-grained;
- medium grain;
- fine-grained;
- fine-grained.
The elements of the first reach a diameter of 3,000 microns. If we are talking about a medium-grained variety, then the grain size is 500 microns. There is a variety of fine-grained graphite grade MPG with a grain size of up to 50 microns. There is also a fine-grained isotropic mineral of the MIG-1 brand, the particles of which have sizes from 30 to 150 microns. Fine-grained graphite and isostatic graphite have grains up to 30 microns in size, their minimum diameter is 1 micron.
Melting temperature
The range of temperatures at which graphite can melt is very diverse. Much depends, for example, on the final objectives of the operation. The temperature range is also determined by external conditions, the characteristics of the composition of a particular mineral, and the use of additional means of influencing graphite during heat treatment. The melting temperature at which it is possible to obtain graphite ready for use varies from 2600 to 3800 °C. Calculation on the Kelvin scale is also practiced. In this case, it already reaches 4000° K, but this value can also increase depending on the pressure indicator. Typically, graphite is melted under pressure of 105 – 130 Bar.
Use of artificial graphite
You already know the density of graphite. However, it is also important to study the area of use of the artificial variety. It is used in all industries. Electrodes are made from coarse grains. Fine-grained structural material is used for the production of shaped products that have complex shapes.
The use of artificial mineral made it possible to achieve high precision in the manufacture of parts. Today, equipment is being produced that fully meets the standards of the present century.
Boiling temperature
The need for heat treatment is driven by the fact that enterprises seek to modify the performance properties of the material in order to create more efficient products. Methods of bringing the mineral to a boil are less commonly used, but they can also improve certain properties of the structure. The question of what is the melting point and boiling point of graphite often involves indicating the same range - from 3800 to 4200 ° C. The lower threshold determines the melting state, and the upper threshold determines the boiling state of the material. Again, depending on the characteristics of graphite and its variety, the conditions of thermal action in terms of obtaining the desired state of the mineral - boiling or melting - may converge.
More information on density and thermal expansion
Depending on the additive, the highest density of graphite can be 5 g/cm3. The minimum value is 2. It is inherent in recrystallized graphite. Single crystals have high anisotropy, this is due to the structure of the crystal lattice. In the basal planes, thermal expansion is negative up to 427 °C. This indicates that the mineral is shrinking.
With increasing temperature, its absolute value decreases. At the above temperature level, thermal expansion is positive. It is directed perpendicular to the base planes. The temperature coefficient of expansion is almost independent of temperature and exceeds the value by more than 20 times compared to the average absolute coefficient for the basal planes.
ELECTRICAL CONDUCTIVITY OF MINERAL
Graphite is an excellent conductor of electricity - in this indicator it is superior, for example, to mercury. Heating the mineral helps improve the conductivity of electrical current. Thus, the mineral has a negative temperature coefficient of resistance. At 0 degrees it is in the range of 0.39-0.602 ohms. As for the resistivity limit, it is the same for all types of material and is 0.0075 Ohm. These properties explain the widespread use of graphite in electrometallurgy.
Structure
When considering what density graphite has, as well as its properties and types, it is necessary to pay attention to its structure. This is a layered substance. Its carbon atoms are arranged in a crystal lattice, similar to a honeycomb. Hexagons in one layer fit tightly to each other. However, the connection between each level is weak. It is this feature that makes graphite easy to break.
According to the Mohs scale, the hardness of the material is equal to one. For comparison, for diamond this indicator is 10, and for porcelain stoneware – 5. At a temperature of 1500°C, according to scientists’ research, the crystal lattice of graphite can be transformed into diamond.
During industrial processing, the structure of the substance changes. At the same time, different grades of graphite have different properties. If the extracted material has not been processed artificially, it is a natural type of substance.
Examples of problem solving
| Did you like the site? Tell your friends! |
News & Events
"PKNM" was provided with preferential financing from one of the largest banks in the country
VTB Bank in Perm within the framework of the Program to stimulate lending to small and medium-sized businesses (“Program 1706”), implemented by the Ministry of Economic Development ...
The petrochemical cluster approved the composition of the Association's board
The next General Meeting of members of the Association for Assistance to the Development of the Petrochemical Industrial Cluster of the Omsk Region was held. The participants elected the Chairman of the General Meeting, his...
Research and production enterprise "POLIPLASTIC" enters the European market
On June 27, 2022, the first International Exhibition Compounding World Expo 2022 will begin its work in Essen (Germany). The event promises to be the largest event for suppliers of…
The testing laboratory of the Novocherkassk enterprise of the Titan Group of Companies has been replenished with new equipment
In June, the industrial testing laboratory of the Novocherkassk Lubricants Plant (part of the Group) was replenished with a new domestic Engler viscometer...
analyzed the results of polypropylene sales for the first five months
(a joint venture of Titan Group of Companies, Gazprom Neft and SIBUR) analyzed the results of polypropylene sales for the first five months of 2022. Sales volume in Russia...
Research and production enterprise "POLIPLASTIC" shared its experience in digitalization of production processes
Evgeniy Ambrosov, technical director of the Research and Production Enterprise “POLIPLASTIC”, took part in the round table “Chemical and petrochemical industry of Russia in the era...
Information
Cast iron pipes with nodular graphite
“PKNM” was provided with preferential financing from one of the largest banks in the country The petrochemical cluster approved the composition of the board of the Association The scientific and production enterprise “POLIPLASTIC” enters the European market
Atg graphite
“PKNM” was provided with preferential financing from one of the largest banks in the country The petrochemical cluster approved the composition of the board of the Association The scientific and production enterprise “POLIPLASTIC” enters the European market
Catalog of organizations and enterprises
Graphite-USSO Graphite products
Stalex
Special steels and materials, custom production, graphite...
Chelyabinsk Electrode Plant
Industry: Not known Criminal Code: 5508.96 thousand rubles. Address: 454038, Chelyabinsk Electrotechnical Plant, Chelyabinsk, Chelyabinsk region. Manufactured products: artificial graphite; polyacrylonitrile (carbon) harness; products made from artificial...
OOPS
CJSC UglerodPromServis is a large producer of graphite and carbon-containing materials in the Ural region. We produce graphite according to specified characteristics and requirements...
GraphitePro
Graphite. Graphite products.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high temperature resistance of graphite (in the absence of oxygen), on its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost any aggressive aqueous solutions (much higher than that of noble metals). For the production of chemically active metals by electrolysis of molten compounds, solid lubricants, in combined liquid and paste lubricants, plastic filler.
It is a neutron moderator in nuclear reactors, a component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin). Used to produce synthetic diamonds, as a nanometer length standard for calibrating scanners of a scanning tunneling microscope and atomic force microscope, for the manufacture of contact brushes and current collectors for a variety of electrical machines, electric vehicles and overhead cranes with trolley power, powerful rheostats, as well as others devices that require reliable moving electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
Graphite – C
| Molecular weight | 12.01 g/mol |
| origin of name | from ancient Greek γράφω - to record, to write |
| IMA status | valid, first described before 1959 (before IMA) |
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, and usually have an imperfect lamellar shape. More often it is represented by leaves without crystallographic outlines and their aggregates. Forms continuous cryptocrystalline, foliated or rounded radial-radiating aggregates, less often – spherulitic aggregates of a concentric-zonal structure. Coarse-crystalline precipitates often exhibit triangular shading on the (0001) planes.