Solder is a metal alloy intended for joining parts and assemblies by soldering. It must have good fluidity in the molten state, well wet the surfaces of the materials being joined, and in the solid state have the required mechanical strength, resistance to the external environment, the required coefficient of thermal expansion, etc.
Solder is selected depending on the type of metals or alloys being joined, the size of the parts, the required mechanical strength and corrosion resistance. For soldering thick wires, solders with a higher melting point are used than for soldering thin wires. In some cases, it is necessary to take into account the electrical conductivity of the solder.
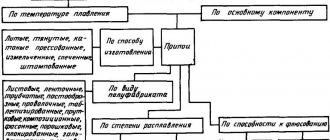
Solders are divided into soft solders with a melting point below 400 °C and hard solders with a melting point above 500 °C. Brazing alloys have higher tensile strength. These include mainly copper-zinc (PMC) and silver (PSr) solders. The main characteristics of solders and their scope of application are given in the table below.
In the radio engineering industry and amateur radio practice, tin-lead solders are most widely used. They are divided into antimony-free, containing no more than 0.05% antimony, low-antimony, containing 0.05...0.5% antimony, and antimony, containing 0.5...6% antimony (GOST 21930-76). Low antimony solders are recommended for soldering zinc and galvanized parts, while antimony solders are recommended mainly for soldering steel parts.
Currently, they are mostly “buying”. There were times when they often got into trouble. Today, the difficulty lies in choosing from the variety of fluxes and all kinds of solders presented on the windows of specialized stores. In the past, it was often easier to “get” the necessary components and prepare the solder yourself.
Let's look at one of the recipes below...
To make solder yourself, carefully dried components of the composition are weighed on technical scales, the mixture is melted in a metal crucible over a gas burner and, after mixing the melt with a soft wood or steel rod, the slag film is removed from the surface of the melt with a steel plate. Then the melt is carefully poured into trough molds made of tin, duralumin or gypsum. Melting must be performed in a well-ventilated area, wearing safety glasses, gloves and an apron made of coarse fabric.
Soldering of various metals and alloys
Products that have been cleaned and prepared for soldering should not be stored for a long time to avoid oxidation.
They should be loaded into an oven or container as soon as possible in a protective environment. Particular attention should be paid to removing air when soldering high-alloy steels and alloys containing easily oxidized elements. Air removal can be achieved by evacuation or purging of protective gas - argon. When blowing, the temperature should increase gradually, starting from room temperature to 800-900 C (1073-1173 K). This process requires significant consumption of argon. Evacuation is more rational, since it significantly reduces the consumption of argon. When soldering, controlling the heating temperature of the product is of great importance; overheating can have a harmful effect. The total time the solder remains in the molten state consists of the time:
t = t1 + t2 + t3
where t1 is the heating time from the melting temperature of the solder to the soldering temperature; t2 is the holding time for soldering; t3 is the cooling time from the soldering temperature to the crystallization temperature of the solder.
In the case of interaction of solder with the base metal, t1 should possibly be reduced. After the punk process is completed, it is necessary to remove the flux, clean the oxidized surfaces, eliminate sagging and areas of solder spreading, especially in those places that are subject to subsequent processing. The requirement to remove flux is caused by its possible negative impact, for example, the appearance of corrosion (in aluminum alloys).
Fluxes (for soldering aluminum alloy) are removed by washing with hot and cold water, subject to subsequent treatment in a solution of chromic anhydride. Borax-based fluxes form a hard crust on the surface. They are removed mechanically or by immersing parts in hot water. Brazed seams on aluminum alloys are treated with a metal brush and washed a second time to remove any fluxes that may remain in the pores of the seams. Spreading solder is removed by mechanical, chemical or electromechanical methods.
Various methods are used to control the quality of solder joints. External inspection of the seams is essential. The seams are checked for strength, density, and electrical conductivity. Soldered seams can be controlled by physical methods: X-ray scanning, the use of radioactive isotopes, and sounding.
In addition to testing soldered samples without destroying them, tests are often used to bring them to destruction. The results obtained from tests to failure of several samples make it possible to establish the mechanical properties of a series of similar products.
Carbon and low-alloy steels include steels with a melting point of 1450-1520 C (1723-1793 K). When low-temperature soldering of steels, mainly tin-lead solders with active fluxes are used. It is recommended to tin the parts before soldering. This speeds up the soldering process and allows for high mechanical properties of the joints.
More often, high-temperature copper-zinc solders with the addition of silver are used for soldering steels (melting point 940-700 C (1213-973 K). However, due to the easy evaporation of zinc, these solders are not used for vacuum punk. It is advisable to use them when soldering in an environment with low temperatures oxidizing properties, for example, products of incomplete combustion of a nitrogen-hydrogen mixture with flux in the form of borax, boric anhydride, etc. For soldering carbon steels, pure copper is also used as solder, especially when soldering in furnaces in a hydrogen environment. Copper spreads well, fills small gaps, while the strength of the connections exceeds the strength of copper itself.
Highly alloyed alloys include corrosion-resistant austenitic steels 0Х18Н9, 12Х18Н9 with stabilizing additives - titanium, vanadium, niobium, etc., acid-resistant chromium steels Х17, Х25 and others of the ferritic class, heat-resistant nickel alloys, for example, having about 80% Ni and etc.
These alloys can be soldered with low-melting solders using active fluxes. However, soldering of this group of alloys with low-melting solders is technically impractical. It is more rational to use high-temperature solders for their connections (Table 1).
Depending on the brand of solder, fluxes with different components are used. Some solders with rapid heating of the heating element. hours lose their components.
High-alloy alloys and steels can be soldered in argon, hydrogen, or in vacuum furnaces. The disadvantage of soldering in argon is the not entirely satisfactory spread of solder. To improve spreadability, additives, such as lithium, are added to fluxes. Soldering in a hydrogen atmosphere requires high purity; The use of hydrogen always involves some risk of explosion.
Soldering in a vacuum gives good results when using solders that do not contain easily evaporating elements (zinc, etc.). When soldering the above materials, pores may appear due to the evaporation of some components of the solder, for example, zinc: lack of penetration as a result of unsatisfactory wetting of the joined parts with molten solder or insufficient cleaning of surfaces; cracks when liquid solder penetrates between the grain boundaries of the base metal. Cracks form especially often when soldering with copper-zinc and copper-silver solders. By using higher temperature solders, cracking of solder joints can be avoided.
Table 1. Composition of solders, %
The use of nickel solders is sometimes accompanied by the formation of undercuts in the base metal at the transition points to the seams. This is due to the fact that this type of solder has the ability to dissolve the base metal. To avoid this phenomenon, the soldering process should be carried out at the lowest possible temperature. By means of soldering, products made of pure copper and copper alloys are well connected. Pure copper solders well when heated in vacuum furnaces, as well as in an atmosphere of well-purified hydrogen without any oxygen impurities. Copper-zinc alloys containing 4-38% Zn lose it when heated for a long time (zinc evaporates), so it is advisable to coat brass parts with copper before soldering.
Soldering is widely used for joining various bronzes; aluminum containing 5-10% Al; beryllium, used in instrument making and containing 2-2.5% Be; chromium, containing about 0.5% Cr; tin, used in pressure treatment, containing tin, as well as phosphorus, etc.
Copper and its alloys are easily soldered using low-temperature solders using rosin fluxes that do not cause corrosion. Often, before soldering, the surfaces of parts are tinned with a layer of pure tin with a thickness of 0.005 mm on steel and 0.0075 mm on copper. Low-temperature solders do not provide high strength solder joints, so soldering in ovens with high-temperature hard solders is recommended. It is advisable to use copper-phosphorus and silver solders and fluxes based on borax with the addition of fluoride compounds. Aluminum bronzes are well soldered with silver solders with nickel, which prevents the penetration of aluminum into the solder and increases the productivity of the technological process.
Titanium and its alloys are soldered in electric furnaces, i.e. h., gas-flame burners. The best mechanical properties of the junction are achieved when soldering HDTV. This is explained by the fact that as a result of the shortening of the thermal cycle with this soldering method, there is no grain growth, leading to brittleness of the joints. When soldering titanium alloys, it is advisable to use silver solders having a melting point below the recrystallization temperature of titanium and above the temperature required to satisfy the conditions for wetting the soldered parts with solder.
A very important production task is the connection of various types of ceramic materials and oxides with each other and with metals by soldering. Different cases are possible: metals are more refractory than ceramics, while the connection of both parts occurs in a solid state, contact is ensured by the necessary pressure and the use of coatings. In the latter case, the connection is achieved at temperatures below the melting point of each of the parts being connected.
Particularly favorable conditions for bonding are when metals have a melting point lower than the melting point of ceramics and, as a result of their specific chemical properties, are prone to form a bond with the latter. For example, titanium and zirconium have a high affinity for oxygen and form solid solutions with many metals and oxides. Oxides of titanium and zirconium are very refractory. Under certain conditions, these metals reduce the oxides of the metals that form the ceramics and absorb the released oxygen. This reduction required for press brazing should be carried out under vacuum or argon conditions.
A serious difficulty in soldering ceramics with metals is the significant difference in their temperature expansion coefficients, as a result of which residual stresses of significant magnitude are formed in the joints. In unfavorable cases, when the materials have insufficient plasticity, cracks appear in them. To eliminate this phenomenon, sometimes plates of ductile metal, such as molybdenum, are placed between the metal being joined and the ceramic. With plastic deformations of the latter, the risk of cracks in ceramics is significantly reduced.
With the help of special filler metals, it is possible to obtain high-quality compounds not only of homogeneous elements, for example Al2O3 + Al2O3, but also of dissimilar ones. Alloys containing strong carbide-forming elements - molybdenum, tantalum, titanium, zirconium, etc. - wet graphite well.
Neutral substances
Neutral fluxes include rosin, which is recommended for soldering small radio components and microcircuits.
This popular reagent is needed in order to solder parts of heterogeneous structure made of copper and its alloys at relatively low heating temperatures at the joint (no more than 450 degrees). Moreover, this operation is permissible even if there are thin oxide films on the surface of the workpiece.
Due to their low activity, rosin-based fluxes ensure protection of products from corrosion and are therefore in high demand.
When preparing the working composition, alcohol, glycerin or turpentine are added to the rosin crushed to a powder state, which helps to improve the quality of the mixture.
Neutral flux gels are sold, which are needed for lead-free soldering of microcircuits. They are convenient to apply with a special dispenser syringe.
Solders and fluxes for soldering
Most soldering methods are carried out using various solders, and only in cases where low-melting eutectics can form between metals during the soldering process is soldering possible without special solder. There are a number of general requirements for solders. Solder should spread well over the surface of the base metal, wet and dissolve it, easily fill the gaps between parts, provide the necessary strength of the connection, etc. Solders are used in the form of tapes, pastes, and rods. Particularly common are solders in the form of wire loops and foil spacers, stamped to match the surface of the parts being joined. High-temperature solders are widely used as solders - alloys based on silver, aluminum, copper, etc., which, as a rule, have a melting point above 450-500 C (723-773 K). Copper-zinc solders PMC 36, PMC 48, PMC 54 have a tensile strength σв = 21-35 kgf/mm2 (206.0-343.2 MN/m3), relative elongation up to 26%, recommended for soldering copper products, tombac , brass, bronze. Silver solders have a melting point of 740-830 C (413-1103 K). According to GOST 8190-56, solder grades are divided depending on the silver content in the alloys, which varies from 10 (PSr 10) to 72% (PSr 72). They also contain zinc, copper and a small amount of lead. These solders are used for soldering thin parts, connecting copper wires, and in cases where the solder location should not sharply reduce the electrical conductivity of the butt joints. Low-temperature solders have a melting point below 450-400 C (723-673 K). They have little strength. They are used for soldering almost all metals and alloys in their various combinations. In most cases, low temperature solders contain a significant percentage of tin. Low-temperature tin-lead solders (GOST 1499-70) have an upper critical melting point of 209-327 ° C (482-600 K). Tin has a melting point of 232 C (505 K). Its tensile strength is 1.9 kgf/mm2 (18.6 MN/m2), relative elongation 49%, HB 6.2 kgf/mm2 (60.8 MN/m2). Tin-lead solders POS-90, POS-61, POS-40, etc. are used for soldering copper devices, aircraft radiators, brass and iron products, copper wires, etc. The formation of a high-quality solder joint largely depends on the possibility the most complete removal of oxide, adsorbed gas and liquid films from the metal surface. In soldering practice, various types of fluxes, a reducing atmosphere or vacuum are used to remove surface films. Recently, mechanical destruction of films using ultrasonic elastic vibrations has been successfully used for this purpose. Soldering fluxes have several purposes. They protect the base metal and solders from oxidation, dissolve or reduce formed oxides, improve surface wetting, and promote the spreading of solders. Fluxes can be used in solid, liquid and gaseous form (in the form of powders, pastes, gas solutions). The role of flux is performed by some special gas atmospheres and vacuum, which can also help restore oxides and improve wetting conditions. In some cases, the fluxing effect is exerted by individual components included in the solder. For example, phosphorous solders do not require fluxes when soldering copper alloys.
Wire with rosin
Combining two consumables in one product significantly simplifies the procedure and improves the efficiency and effectiveness of the work.
If rosin as a flux is added separately, there is a high probability of adding an excess amount. In the finished wire with flux, the ratio is fixed, stipulated by GOST.
Most often, products are supplied in a coil or coil. The version with wire packaging in coils is intended for continuous operation on an industrial scale. The assortment includes products of a wide variety of thicknesses.
Products in the form of coils are used for slightly smaller scales of use.
This option is suitable for both individual craftsmen and repair businesses. The thickness of the wire packaged in coils varies from 0.8 mm to the maximum possible 2 mm.
The solder component is an alloy of 2/5 parts lead and 3/5 parts tin. Each solder granule is surrounded by rosin, the total concentration of which varies from 0.8% to 1.2%.
The convenience of the wire is due to its flexibility . When soldering, such a mixture can be easily introduced into any gap, where it will melt and provide good envelopment of the parts and the formation of a strong seam.
The disadvantage is the low melting point of the mixture . Parts made from refractory alloys cannot be soldered with such consumables.
Working with solder wire containing rosin flux is similar to standard soldering. First, the parts need to be cleaned, then heated to the required temperature and consumables introduced into the work area.
Ceramic fluxes.
They are made in the same way as electrode coating.
The dry components of the charge are mixed in liquid glass. The resulting mass is crushed by pressing. Then they are calcined and sifted to obtain particles of a certain size. The dry mixture particles can be held together by sintering. This happens at elevated temperatures without melting. Then granulate to the required size.
Non-melting fluxes are prepared in the form of a mechanical mixture. The most common are ceramic fluxes. The composition is close to the composition of the main coating. Alloying metal with flux is achieved by introducing ferroalloys into their composition. The combination of alloying elements can be different, and this makes it possible to obtain almost any composition of the weld metal.
This is the most characteristic feature of ceramic fluxes.
The chemical composition of the weld also depends on the welding parameters.
To determine how the properties of the seam have changed, it is necessary to measure the hardness in various places.
The most critical zone is the fusion zone and the heat-affected zone. Ceramic fluxes also have their disadvantages: low strength, as a result of which their granulation changes during transportation or operation.
Often used for welding high-alloy and special steels, as well as for surfacing work.
Types of solders
All existing solders can be divided into three main groups:
- Refractory solders;
- Low-melting;
- Ultra-low-melting.
Refractory solders are not suitable for soldering radio components, since melting them requires a fairly high temperature, over 500 degrees. Refractory solders are capable of creating a very reliable connection of metals, which is characterized by a gap of at least 50/kg per 1 mm².
An electric soldering iron is not suitable for soldering with refractory solders, since it is not able to provide the required melting temperature. More powerful tools are already used here, for example, a gas burner.
Amateur radio solders, also known as light alloys, contain tin and lead. They are soft and melt well at temperatures up to 200 degrees. Therefore, most often it is light-alloy solders that are used for soldering radio components.
Melting fluxes.
Alloys of metal oxides and salts. The process of their manufacture includes the following stages:
1. Calculation and preparation of the charge. 2. Melting of flux. 3. Granulation. 4. Drying if wet granulation was used. 5. Sifting.
Pre-crushed parts of the flux are loaded into arc or melting furnaces. After melting and holding until the end of the reaction at a temperature of 1400 C, the flux is released from the furnace.
In dry granulation, flux is poured into metal molds. After cooling, the casting is crushed using rollers. Particle size 0.1-3 mm. The fluxes are then sieved.
Dry granulation is used for hygroscopic fluxes containing large amounts of fluoride and chromium salts.
The advantage of these fluxes is that they can be used several times.
Used for welding aluminum and titanium alloys.
Wet granulation method: molten flux is released from the furnace in a fairly thin stream and falls into a container with running water. In some cases, an additional stream of water is used. Next comes sifting.
Various granulations are obtained. The flux is dried at a temperature of 250-300 C, and then crushed, if necessary. After this they sift.
The flux consists of uneven grains of light gray, red-brown and brown color.
Transported in sealed containers, plastic bags, barrels.
Melting flux cannot contain alloying elements in pure form, since they are oxidized during the manufacturing process. Therefore, alloying occurs by reducing flux oxides.
The classification of fluxes by chemical composition is based on the content of oxides and salts in it.
There are oxidizing fluxes containing manganese and silicon oxide in their composition.
To obtain certain properties of the flux, other components are introduced into its composition - fluorspar, more durable oxides.
The more manganese and silicon oxide in the flux, the more it can alloy the metal with these elements, but the more it will oxidize this metal.
Melting fluxes are used for welding carbon and low-alloy steels.
Non-oxidizing fluxes practically do not contain manganese and silicon oxides; they contain fluorides and are used for welding high-alloy steels. Also, non-oxidizing fluxes can consist of fluoride and chloride salts and elements that do not contain oxygen. Used for welding highly active metals - aluminum and titanium.
Due to the widespread use of fluxes, there is a GOST for the main grades: GOST 9087-81 “Welding melting fluxes”. Regulates the chemical composition.
A distinction is made between glassy and pumiceous grains. The grain structure depends on the composition of the flux melt and the degree of its overheating. Depending on this, the flux can be dense, transparent, porous, or loose. It should be taken into account that pumice flux, with the same chemical composition, has one and a half to two times less weight than glassy one.
These fluxes protect the metal less well from exposure to air, but provide good weld formation at high current densities and welding speeds.
Letters in flux designations:
- M – small
- C – glassy
- P – pumice-shaped
- SP – mixed
Soldering through-hole components
1. Install the component into the mounting holes, if necessary, bend the leads.
2. Apply the soldering iron tip in such a way that simultaneous contact is ensured with the mounting hole CP and the component lead, warm up for 0.5-1.0 s.
Read also: Features and choice of ultrasonic washing
Struck No. 1. It is necessary to ensure good thermal contact between the soldering iron tip and the soldered surfaces.
3. Apply a small amount of solder to the soldering iron tip so that a solder bridge is formed between the CP and the terminal (see figure).
4. Move the tubular solder in a circle along the CP in the opposite direction from the soldering iron tip (see figure).
5. Once the solder joint is formed, remove the solder rod.
6. At the same time, remove the soldering iron tip. To form the correct fillet shape, the soldering iron tip must move upward along the component lead.
Rule No. 2. It is necessary to ensure contact between the soldering iron tip and the soldered surfaces until a solder fillet is formed.
Attention! Avoid applying strong pressure with the soldering iron tip to the gearbox. Do not allow the soldering iron tip to contact the solder fillet without using tubular solder, this may cause degradation of the solder joint.
Submerged arc welding
At first glance, it may seem that one of the main advantages of submerged arc welding - the possibility of obtaining a large depth of penetration of the metal being welded - contradicts the conditions for welding thin sheet steel. However, under certain conditions, submerged arc welding allows regulation of the depth of metal penetration, starting from fractions of a millimeter, and therefore its well-known advantages can be used for welding thin sheet steel. The successful introduction into the production of submerged arc welding of thin-sheet steel products became possible mainly due to the use of thin welding wire. There are known examples of welding thin sheet steel and conventional electrode wire with a diameter of, for example, 4 mm. However, in this case it was possible to weld steel with a thickness of at least 3-4 mm, provided that the product was assembled very carefully. For welding thin-sheet steel, it is of great importance to use devices that facilitate the precise assembly of the product and ensure reliable pressing of a copper or flux-copper lining, flux pad, etc. to the welded joint. Experience shows that the productivity of automatic welding of thin-sheet steel products with relatively short seams does not depend as much on the machine welding speed as on the time spent on preparatory and auxiliary operations. Therefore, an important task is to develop effectively operating assembly and assembly-welding devices. The smaller the amount of thermal energy transferred from the arc to the base metal during the welding process, the smaller the depth of its penetration and, therefore, the thinner the metal can be welded without burns. The thermal energy transferred to the base metal can be reduced by reducing the power of the arc or increasing the speed of its movement along the welded joint. For welding thin sheet steel, a reduction in arc power is generally used, rather than an increase in welding speed. This is largely explained by the fact that the use of high welding speeds (more than 150-200 m/hour) is associated with strict requirements for the accuracy of maintaining the welding mode, the need for thorough cleaning of the welded edges, with very precise assembly of joints, in some cases with a special tilt of the product and electrode, etc. At the specified welding speeds, the weld metal may be affected by pores, transverse cracks and other defects. If we take into account that the productivity of welding thin-sheet steel, as indicated above, mainly depends on the time spent on installation and preparatory operations, then it will become clear why increasing the speed has not become the main way to reduce heat input.