Although stainless steel is highly resistant to corrosion, the additional protection provided by passivation is desirable. In some cases, when even products made of stainless steel are at high risk of corrosion, the need to perform such a procedure is beyond doubt.
Examples of stainless steel surfaces subjected to corrosion and the results of passivation
Metal passivation technology, types and compositions
Passivation is the formation of thin oxide or salt films on the metal surface that protect it from external corrosion.
This coating prevents metal from coming into contact with oxygen and aggressive environments. During passivation, protective films can form on a metal surface both naturally and artificially. In the first case, they consist of oxides of chemical elements that are part of the metal itself, and in the second, they may include oxides and salts of other chemical elements. For example, pure aluminum naturally forms a very durable oxide film and is therefore resistant to most types of corrosion. But products made from its alloys, containing chemically active components, already require artificial corrosion protection and therefore undergo passivation in saline solutions. Passivation is widely used to protect the surfaces of products made of steel, copper, nickel, aluminum and their alloys. Even protective zinc and cadmium coatings are passivated with chromium salts to increase their corrosion and mechanical resistance. Passivation of a metal causes the formation of a layer of oxides or salts several microns thick on its surface, which practically does not affect the geometric dimensions of the products. On the other hand, such films can reduce the contact conductivity of the base material, but, as a rule, to a lesser extent than a layer of corroded metal.
Types of corrosion
Despite the fact that the corrosion process leads to almost the same consequences, the reasons that cause it may be different. The most common cause of corrosion of stainless steel products used in domestic conditions is the use of cleaning products containing a significant amount of chlorine in their chemical composition. Such agents actively contribute to the destruction of the oxide film on the metal, which leads to the development of a corrosion process over its entire surface (i.e. general corrosion).
Crevice corrosion of stainless steel occurs in cases where parts made of such metal come into contact with each other for a long time. Corrosion of this type, which is typical, often begins to develop in the fastening areas. There is also pitting corrosion, which is often called pitting. It occurs when the oxide film on stainless steel is damaged mechanically.
Corrosion of stainless steel under water manifests itself to a greater extent at the joints of parts
If stainless steel comes into contact with a dissimilar metal in a conductive environment, corrosion begins to develop, which is called galvanic. Products made of stainless steels that are used in sea water and at the same time in contact with metals with a lower degree of alloying are most susceptible to this process.
Intergranular corrosion is a very common phenomenon that occurs when a stainless steel product has been subjected to significant overheating. With strong heating (over 500°), chromium and iron carbides are formed at the boundaries of the crystal lattice of stainless steel, which cause a decrease in the strength of the metal.
Corrosion of stainless steel can occur due to the use of chlorine-containing cleaning compounds
There is also erosive corrosion, which occurs if stainless steel is constantly exposed to an abrasive environment. Constantly affecting the metal surface, particles of such a medium destroy the protective oxide film, which does not have time to recover.
The essence and description of the metal passivation process
When passivating, the surfaces of metal products are treated with solutions of chemical compounds that have oxidizing properties. This role is most often played by acids, nitrites and solutions of chromium salts (less commonly, molybdenum). The solution is applied to the surface of metal workpieces by immersion or manually using special equipment. Solutions used for passivation usually consist of a main reagent and several additives that accelerate and stabilize the passivation process.
In general, the passivation process consists of the following stages:
- Mechanical cleaning of product surfaces.
- Chemical degreasing in a solution of sodium hydroxide and soda ash.
- Rinse in running hot and then cold water.
- Passivation for a specified time.
- Neutralization in soda ash solution.
- Rinsing by repeated immersion in running cold water.
- Dry in a drying cabinet or blowing warm air.
- Surface quality control after passivation is carried out visually or instrumentally. If the result is unsatisfactory, the passivation process is repeated, starting from step 1.
The given example describes the technological process of passivation using stationary production equipment. To passivate the surfaces of products at the site of their installation, hand-powered tools and devices are used (see photo below).
Properties of passivated metal and its application
After passivation, a corrosion-resistant layer is formed on the metal surface, which, if chromates are used, also has increased mechanical strength. Some metals and alloys are prone to natural passivation. This is especially true for aluminum and stainless steel with the presence of chromium. But if the structure and chemical composition of the surface layer is damaged, they can also be subject to corrosion. When passivating stainless steel, its own chromium is used to create a durable surface protection, which, when combined with oxygen, forms a dense oxide film. All stainless steel products operating in aggressive environments are pre-passivated, which helps to avoid (or delay) their corrosion.
Passivation of iron and its alloys in the form of structural and special steels is usually carried out over a coating of nickel, zinc or cadmium using chromium salts. This passivation strengthens the surface layer and allows steel products to be used for a long period without the danger of corrosion, and if it occurs, only the affected areas should be treated. Passivation of copper and its alloys (bronze and brass) is carried out for both protective and decorative purposes using chromate solutions. In this case, a thin transparent film is formed on the surface of the copper product, protecting the metal from oxidation and preserving its presentation.
Passivation of silver is carried out for the same purposes using similar technologies.
Passivation and care of stainless steel brewing equipment
Despite its reputation as an ideal metal for beer production, stainless steel can cause corrosion or rust. So this week we'll take a look at how and why stainless steel can corrode, as well as how you can passivate your stainless steel brewing equipment to protect it.
Stainless steel and rust
Steel is made from an alloy of iron and carbon, and carbon makes up only half or a little over a percent of its composition. In comparison, stainless steel is made from iron and chromium. Chromium contains approximately 10-30% of steel, and it is an important element that makes stainless steel resistant to corrosion.
The chromium in stainless steel reacts very quickly with oxygen, and actually forms a protective layer of chromium oxide on the surface of the steel. This chromium oxide prevents the formation of rust and corrosion. However, if the chromium layer is compromised for any reason, the iron in the steel can actually begin to corrode and rust.
Your stainless brewing equipment is generally very resistant to corrosion. However, if you expose it to bleach or other bleach cleaners, scratch it, over-clean it, or expose it to regular rusting steel pads or leave it in contact with regular steel, it can damage the protective layer. Bleaching agents can completely remove the protective layer.
Excessive cleaning, especially with steel wool, can also undermine your oxidation layer. It is important to store regular steel in the same place as regular buckets, tools and some equipment other than your stainless steel equipment. Iron from ordinary steel tends to damage stainless steel (a property of iron) and destroy the oxidation layer.
Do not place regular steel buckets or mixed metal tools or equipment in your stainless steel kettle after boiling.
Passivation of stainless steel to protect it
When stainless steel products are made, they are typically immersed in a nitric acid bath at the end of the manufacturing process to remove contaminants. The acid also activates the oxidation process of chromium in the air called passivation, where a protective layer of chromium oxide is formed when oxygen reacts with chromium. Passivation occurs very quickly - usually within 20 minutes.
Now some stainless brewing equipment, particularly lower cost stainless materials, were likely machined, stamped, pickled, polished and welded only after the stainless steel had been fabricated and acid washed. As a result, it may contain oils, polishes, welding compounds and other contaminants that protect the steel but should be washed away the first time you clean your parts. Plus, you probably don't want to find these oils and compounds in your beer.
Step 1: Thorough Cleaning
Since you likely don't have access to a large bath of nitric acid, at-home passivation begins with a very thorough cleaning. If this is new equipment where you want to remove any substances left over from manufacturing and finishing. This will require a strong cleaning agent such as trisodium phosphate (TSP). Mix TSP in the recommended proportion with hot water.
Bar Keeper's Friend is also a good cleaner for stainless steel equipment (Note: it is an imported version similar to Pemolux powder but more effective), although you should not use it on etched metals. They also sell a "soft scrub," a liquid version of Bar Keeper's Friend that is easier to use. Make sure you remove all fittings, valves and other small items and clean them too.
Rinse and dry everything thoroughly after cleaning.
Step 2: Acid passivation
Now that all dirt, oils and impurities have been removed, you can begin the next step of metal passivation. This is achieved by using a weak acid and then air drying the metal. Oxygen contained in the air will interact with chromium, forming a passive protective layer. Always wear gloves when working with these acids as they can cause skin irritation in high concentrations.
There are several options you can use here. One option is to use Bar Keeper's Friend, which contains oxalic acid. It works well on stainless steel, but do not use it if you have had electronic etching applied to the surface of your equipment as it will destroy or even remove the etching.
Add enough water to form a thick paste and apply the product to the object that requires passivation. Let it “sit” on the metal for 5-10 minutes, and then gently wipe it with a dry towel.
Types of passivation
According to the coating application method, passivation is of two types: chemical and electrochemical. In addition, varieties of this technology are classified according to the type of chemical element from the compounds of which the surface film is formed (chromatization, nickel plating, molybdenation, and others). In addition, natural passivation is distinguished - the process of formation of a protective layer in a number of metals and alloys under the influence of atmospheric and dissolved oxygen in water.
Chemical
Chemical passivation occurs as a result of the attraction of negative ions of salts dissolved in water to the surface of the metal, the atoms of which have a positive potential. To do this, metal products, previously cleaned and degreased, are placed in a special bath filled with an appropriate solution. The main component in such an electrolyte is a metal salt that forms a protective film on the surface of the product. Chemical passivation can also be performed at the site where the product is installed. In this case, all processes, from cleaning to passivation, neutralization and washing, are performed manually using special equipment.
Aluminum passivation
On aluminum, an oxide and very durable film is formed under natural conditions under the influence of atmospheric oxygen. Many people remember the school experience when a small layer was removed from an aluminum wire dipped in mercury using a file, and then this file-treated tip was removed from the mercury. And the treated end in air was instantly covered with a “fur coat” of oxide crystals. But under normal atmospheric conditions, oxides do not form on aluminum so quickly and take the form of a transparent film with a thickness of only a few microns. In its properties it is very close to the chemically inert aluminum oxide corundum. The disadvantage of such a natural film is its instability with a significant increase in temperature or with prolonged exposure to active acids.
For lasting protection, one cannot do without the anodizing process, which results in protective films with a thickness of 5 to 20 microns. And in certain modes it is possible to obtain ultra-strong films (withstanding loads of up to 1500 kg per mm, that is, higher than that of tool steel.
Contents of passivation compositions
The composition of solutions for passivation of non-ferrous metals most often includes potassium and sodium chromates, as well as chromic anhydride as the main reagent. To create an acidic environment, various acids and salts are added to such electrolytes, the composition of which affects the speed of creation and uniformity of the protective film. Copper passivation is carried out in solutions containing small amounts of sulfuric acid. When processing aluminum, phosphoric acid is included in the electrolytes, and additives in the form of nitric and sulfuric acids are used to passivate zinc and cadmium. The content of passivating solutions for processing steel products depends on their composition and often includes nitric acid and its salts.
All chromium salts (especially hexavalent) are very toxic. Therefore, chrome passivation of metal products can only be carried out in specialized industries that have appropriate cleaning and drainage systems, as well as specially trained personnel.
Nowhere is it written how passivation with chromium salts is carried out directly at the installation sites of the equipment. How are chemicals removed in these cases? Or are other compounds used for this treatment? If anyone has information on this issue, please share it in the comments to our article.
Passivation of structural and special steels
For reliable passivation of steels, it is advisable to pre-coat them, all or partially (those elements that will experience the greatest impact of adverse factors) with nickel, zinc or cadmium using chromium salts. Passivation with these salts is advantageous in that, after strengthening the surface layer, the products can be used for a very long time without the risk of corrosion. And if individual sections begin to rust, they can be passivated, without disassembling or removing the structure, with the same composition with chromium salts right on the spot, by applying overlays soaked in solutions.
What is passivation?
The passivation process allows stainless steel to return to its original properties, further protecting it from the effects of many external factors. This is a special chemical treatment of metal products, after which a special protective coating is formed on their surface. When interacting with concentrated acids, an inconspicuous film appears on stainless steel. This process is called passivation.
This method is used both for additional processing during the production of products and for restoring the basic properties of stainless steel parts.
History of the discovery of aluminum
For a long time, man has known the compound of the metal in question - potassium alum. It was used as a means that could swell and bind together the components of the mixture; this was also necessary in the manufacture of leather products. The existence of aluminum oxide in its pure form became known in the 18th century, in its second half. However, no pure substance was obtained.
Read also: Star-delta motor connection diagram
The scientist H. K. Ørsted was the first to isolate the metal from its chloride. It was he who treated the salt with potassium amalgam and isolated gray powder from the mixture, which was aluminum in its pure form.
Then it became clear that the chemical properties of aluminum are manifested in its high activity and strong reducing ability. Therefore, no one else worked with him for a long time.
However, in 1854, the Frenchman Deville was able to obtain metal ingots by electrolysis of the melt. This method is still relevant today. Especially mass production of valuable material began in the 20th century, when the problems of generating large amounts of electricity in enterprises were solved.
Today, this metal is one of the most popular and used in construction and the household industry.
Why is this necessary?
A stainless steel sheet has a very thin oxide film on its surface. It is this that prevents the formation of rust on parts, fasteners, and hardware made from this material. But the slightest violation of the integrity of this coating leads to the fact that the main anti-corrosion properties of stainless steel are lost. The causes of damage to the oxide film can be very different:
when the material comes into contact with chlorine; when steel interacts with sea water; in case of mechanical or physical damage, including scratches and minor dents.
Therefore, it is important to comply with the operating conditions that are regulated by the manufacturing plants of certain products (cutlery, fasteners, hardware, working tools, solid sheets, etc.). It is prohibited to use detergents containing chlorine and other aggressive chemicals.
But the greatest damage to the oxide film is caused by welding. This is especially harmful in the case of pipe welding. In such a situation, the protective surface is destroyed along the entire seam. Steel passivation is used to restore surfaces and protect products from rust. But here the composition of the stainless steel plays an equally important role.
First cleaning
- Grease, coolant or other contaminants must be thoroughly removed from the surface to obtain the best corrosion resistance. A commercial degreaser or detergent can be used to clean mechanical oils or coolants. Foreign matter such as thermal oxides may need to be removed by grinding or by methods such as acid etching.
- Sometimes the operator may skip basic cleaning, incorrectly assuming that simply immersing the grease in an acid bath will both clean and passivate simultaneously. This doesn't happen. Instead, the grease contaminant reacts with the acid to form gas bubbles. These bubbles collect on the surface of the workpiece and interfere with passivation.
- Even worse, contamination of the passivation solution, sometimes with high chloride content, can cause a corrosion “flare-up”. Instead of producing the desired oxide film with a shiny, clean, corrosion-resistant surface, flash causes a heavily etched or darkened surface—the deterioration of the surface itself that passivation is designed to optimize.
- Parts made from martensitic stainless steels [which are magnetic, moderately resistant to corrosion, and have a yield strength of up to (1930 MPa)] per square inch are hardened at high temperature and then annealed to provide the required hardness and mechanical properties. Precipitation hardenable alloys (which provide a better combination of strength and corrosion resistance than martensitic grades) can be solution processed, partially processed, held at lower temperatures, and then finished with machining.
- In such cases, the parts must be thoroughly cleaned with a degreaser or cleaner to remove traces of cutting fluid before heat treatment. Otherwise, the cutting fluid remaining on the parts will cause excessive oxidation. This condition can cause the underlying layers to remain mottled even after descaling by acid or abrasive methods. Cutting fluids may remain on the parts and harden in a vacuum oven or protective atmosphere, and carburization of the surface may occur, resulting in loss of corrosion resistance.
Etching
- Pickling is the removal of the adjacent low chromium metal layer from the surface of stainless steel by chemical means.
- Where steel is heated by welding, heat treatment or other means to the point that a colored oxide layer can be seen, there is a chromium-depleted layer on the surface of the steel below the oxide layer. Lower chromium content gives lower corrosion resistance. To restore the best corrosion resistance, the damaged metal layer must be removed, exposing the fully alloyed stainless steel surface. Mechanical removal may produce abrasive or other particles (corrosion inhibitors) or may be impractical, so chemicals are usually used.
- Procedures involving etching solutions of nitric (HNO3) and hydrofluoric (HF) acids remove the scale and chromium-depleted underlayer and restore corrosion resistance. Etching solutions also remove contaminants such as iron and iron particles. Etching solutions other than mixtures of nitric and hydrofluoric acids exist and can be used for specialized applications.
- Etching pastes, where a solution is mixed with an inert carrier, are typically used to treat selected areas such as welds.
- Etching involves the removal of metal and a change in the visual brightness of the metal.
- Electropolishing is a useful alternative to etching. Metal removal is achieved but usually results in a bright, smooth and more corrosion resistant surface.
Stainless steel classification
The anti-corrosion properties of stainless steel directly depend on its composition. Based on this, this steel is marked. The classification allows you to distinguish each type of stainless metal by flexibility, hardness, and degree of anti-corrosion protection. Depending on the composition and purpose, they are distinguished:
martensitic steels. Knives (including those for the food industry) and turbines are usually made from them. This steel, having a large percentage of chromium in its content, is very hard; ferritic materials. The amount of chromium in such steel exceeds the previous value by 3-4%. This material has high resistance to phosphoric acid, ammonium nitrate and nitric acid; austenitic steels. This type of stainless steel is very ductile. It is often used in mechanical engineering; duplex or ferro-austenitic metals. These are very durable, but at the same time flexible stainless materials.
Based on the composition of the stainless steel, you can determine whether there is a need for additional processing of the products or not. The likelihood of corrosion on the surface of elements made from this type of steel also depends on this.
Causes of corrosion
Despite the fact that the chemical composition of stainless steel must contain passivators that significantly increase its corrosion resistance, its surface and internal structure can be subject to corrosion.
The main reason why stainless steel begins to deteriorate is insufficient or uneven chromium content in its chemical composition. Contact with metal, which is significantly less resistant to oxidation, can also cause corrosion. Stainless steel products that were connected to each other using welding technology are often subject to destruction.
Corrosion of heated towel rail pipes caused by dishonest welding by the manufacturer
Technology and methods
There are various methods for processing stainless steel. But there are two main methods of steel passivation:
Etching with chemical acids (concentrates) in certain areas. This technology is often used for processing welds, but is also allowed in other cases. This process has different processing sequence options. They differ both in the composition of chemicals and in the time of work. The most common method in this case is electrolytic etching. This technology consists of placing a stainless steel product in a specially prepared bath consisting of concentrated acids. An electric current (alternating or direct) is passed through this composition. The metal plays the role of either a cathode or anode. The supplied current has a mechanical effect on the steel, resulting in the release of hydrogen or oxygen gas. This helps to separate the oxide film on the surface of the product. Etching with ready-made acid mixtures. They can be made in the form of pastes, gels, sprays, concentrates. This method is the most convenient.
Regardless of which method is used to passivate stainless steel, it is important to follow the sequence of work.
Stainless steel and rust. Passivation
We all know about the existence of stainless steel, but not everyone knows its composition. Thus, ordinary steel consists of iron alloyed with carbon, and its percentage usually does not exceed one. As for stainless steel, in addition to iron, chromium can be found in its composition. It accounts for approximately 10 to 30%.
Chromium is the very element that protects stainless steel from corrosion. How does this happen? Chromium reacts with oxygen, thus creating a protective layer on the surface (chromium oxide). It is this that protects the product from corrosion and rust.
But do not forget that the protective layer does not give a 100% result, because it can be damaged, which leads to a weakening of the protection, and, consequently, to corrosion with rust.
How can you destroy the protective layer? There are several ways:
1. Bleach (and other cleaning bleaches). With these materials you can completely destroy the protective layer;
2. Scratches;
3. Excessive cleaning;
4. Impact of steel jaws;
5. Contact with steel susceptible to rust. Ordinary iron can damage stainless steel due to its properties, thus destroying the protective layer;
Passivation of stainless steel to protect it
Many people are interested in how stainless steel products are made. In this article we will answer this question.
To begin with, it should be noted that at the end of production, the products are placed in nitric acid to get rid of various contaminants. This also promotes the oxidation of chromium in the air. This process is called passivation. It lasts about 20 minutes.
Of course, it is always possible to find cheaper equipment, but in this case there is a possibility that the product will not be completely cleaned and washed. Therefore, sometimes it is better to pay a slightly higher price, but be sure that stainless steel products, especially if you plan to use them in the food industry, have been fully processed, cleaned and protected.
Now let's talk in more detail about passivation at home:
1. Cleaning. First of all, the product undergoes scrupulous cleaning, especially if you do not have the opportunity to use nitric acid. But what to use? A strong cleaning agent (for example, sodium triphosphate) is suitable for this. It must be mixed with hot water. It is necessary to clean not only the surface of the product, but also small parts. After cleaning, rinse and dry the product.
2. Passivation using acid. After removing dirt, oils and various impurities, the next step of passivation begins. A weak acid is used for this. For example, citric acid. You should mix it with warm water. A 4-10% concentration is sufficient. It is necessary to keep the product in this solution for about 30 minutes. After this, the product must be left to air dry (preferably overnight) so that the chromium reacts with oxygen and forms a protective layer.
Important!
Do not forget about your safety when working with acids! Be sure to use gloves to protect your skin from irritation.
Stages of chemical passivation
In the process of forming a homogeneous inert film on the surface of stainless steel products, it is important to take into account the characteristics of the steel composition and the degree of damage to the protective coating. Chemical passivation today is an integral part of working with stainless materials. This allows you to extend their service life, get rid of rust and damage, and prevent the formation of corrosion. During passivation work, the following sequence of steps should be followed:
First, the materials are cleaned from contaminants. Grease stains, rust and other deposits are removed. With chemical acid etching technology, the product is immersed in a bath with a mixture of hydrochloric acid and sulfuric acid. At temperatures from 60 to 80 degrees, the steel is kept here for 20-40 minutes. If the method of etching with ready-made mixtures of acids is used, then special concentrated compositions (pastes, gels, sprays) are used for cleaning, which are applied to the surface of the steel manually. The chemical is left for approximately 30 minutes. Then the products are thoroughly washed with water. The passivation process begins. In the first case, the steel is immersed in an acid bath. In the second, gels, pastes, sprays and other ready-made chemical compositions are applied to the surface of the product. In the case of ready-made products, one more stage is provided - treatment with a passivator. This allows for the forced formation of an oxide film on stainless steel. The last stage consists of thoroughly washing the product.
The composition of stainless steel and the grade play an important role in the appearance of the product after chemical passivation. Some species are dark in color, while others are lighter. But regardless of this, this method of steel processing has a whole list of advantages:
improves resistance to corrosion; the surface of the product is uniformly smoothed; burrs, scratches, dents are removed; The service life of the products is significantly increased.
Passivation of silver
Silver is a noble metal, despite changes in its properties in the light (it darkens). Before the advent of digital photography, this ability of silver was used in the creation of light-sensitive materials (film and photographic paper).
But the darkening of silver products in everyday life is often an undesirable process, and to prevent it, chemical methods are used to protect the upper layer of metal, bordering with air, from exposure to light and air. The best way to prevent such changes is passivation by treating silver in a chromium peak - potassium dichromate K2 Cr2 O7.
To carry it out, 60 g of chrompic is diluted in 1 liter of boiled, soft water. The working temperature of the solution is from 25 to 40 degrees, this is not critical. Passivation is carried out by simply immersing the silver item in the bath completely for 20 minutes and periodically stirring the solution. In cases where the diluted amount of chromium does not completely cover the product (a figurine of a complex shape or a voluminous silver candelabra), it is better not to practice alternating surface treatment in parts, but to dilute the reagent in the amount of water required for the normal volume.
Essence of the process
Passivation is not an electrolytic finishing operation that increases the corrosion resistance of stainless steels. The passivation process typically uses dilute nitric or citric acid to promote the formation of an inert protective oxide layer. It is more inert to air, so it slows down subsequent corrosion.
The acid chemically removes—dissolves—free iron from the stainless steel surface, replacing it with a thin surface film of less reactive oxides. Since any stainless steel contains a large amount of chromium, passivation results in the formation of chromium oxide, which has an increased thickness. The surface is passivated and rust protection is improved. At the same time, surface contaminants are removed.
Reasons for metal stability
The corrosion process is characterized by the fact that, gradually oxidizing under the influence of negative factors, the surface of stainless steel is destroyed. If no measures are taken, the destruction will affect its deeper layers.
Table of stability of metals in different environments
Passivation of the metal avoids this problem. The surface of the product is covered with a protective oxide film, and special additives included in the treatment solution improve the properties of the stainless steel. The new material is not damaged.
In industrial conditions, it is possible to obtain a layer of corrosion protection that is ideal in thickness and uniformity. If the conditions in which the product will be used are not too aggressive, then it does not need additional processing. It is important to remember that mechanical damage to steel gives impetus to corrosion processes.
What oxidizing agents are required for passivation?
The main condition for passivation of stainless steel is that passivation does not destroy the base metal. Therefore, the oxidizing agent must be “soft”, with a relatively low pH. Under such conditions, a protective passive film forms spontaneously. It is better to use citric acid as such substances, since organic acids work more gently than mineral ones, and they do not require special preparation.
Is it possible to do without passivation? Stainless steel has corrosion-resistant properties due to its chromium content, but is not completely impervious to corrosion. Oxidizing in the presence of citric acid, chromium forms a moisture-resistant surface film.
Passivation of steel
Iron, which is part of any type of steel, as its basis, is subject to corrosion more than any other metal. The best protection against corrosion for iron-containing materials is the addition of alloying additives to the iron melt, which make the steel stainless. But stainless steel is expensive. Therefore, simpler grades of steel can be protected from rust by treating them in electrolytic baths with the addition of inhibitory pigments in the form of red lead - iron or lead - to the electrolyte.
| These pigments can also work as chemical passivators, without the use of a complex mechanism for connecting them with the metal being coated. The application of such pigments is carried out with ordinary painting supplies, and is usually associated with the large dimensions of the treated surfaces, which cannot be placed in an electrolytic bath (hulls of all types of ships). But in this case the protective effect will be weaker. |
During anodic coating with the help of pigments in the boundary treated outer layer, a high current density occurs in the pores of the formed protective film. In iron, as part of a steel alloy, protective oxide films cannot be formed under natural conditions, so passivation is possible only if inhibitor pigments are included in the coating mechanism.
But the main difference in the formation of protective layers on metal by chemical and electrolytic passivation methods lies in the speed of the process and the strength of the formed phase film. After all, both in a chemical bath and in it, but with electric current and voltage added to the process, the process of formation of an oxide or salt film follows the same scenario.
Sequence of passivation
The following procedure for carrying out the technology under consideration is recommended:
- Preliminary cleaning of the surface of the part to be passivated from any contamination.
- Chemical treatment by immersing the material in a bath of citric acid.
- Washing in water.
- Neutralization of acid residues in an aqueous solution of sodium carbonate.
- Drying.
- Testing the finished surface (the electrical contact measurement method is used, since the conductivity of the passivated layer is worse than that of a conventional one).
Passivation is recommended for all grades of stainless steel that contain more than 0.02% sulfur (even if the surface visually appears clean and shiny). Particularly desirable is the processing of steels containing sulfides, as well as titanium and tantalum - metals whose oxides are relatively quickly destroyed in a humid atmosphere.
To enhance the efficiency of passivation, sodium dichromate is usually added to acid bath solutions. Options with the simultaneous application of ultrasonic vibrations are more productive: under such conditions, the formation of chromium oxide is intensified, which begins even when the material being processed is in an acid bath.
The thickness of the passivating film is very small - up to 5 microns, but this is enough to reliably protect the stainless steel surface from corrosion.
Etching process
Before etching, it is necessary to thoroughly clean and degrease the metal surface from foreign substances such as grease, oil, adhesives, rust, etc. Surface cleaning can be done with any cleaner, including alkaline cleaners, acidic cleaners, and solvent-based cleaners. The correct cleaning solution is selected based on several factors:
Material and configuration of equipment/parts Level and composition of pollutants
After cleaning and degreasing, the cleaning solution is washed off the surface and etching is carried out using one of the methods mentioned above. Process control is very important as corrosion and pitting can occur if the acid concentration is too high and/or if the acid contact time is too long. Once the process is complete, be sure to ensure that all residual acids are removed and neutralized to prevent pitting and pitting. To achieve optimal corrosion resistance, it is recommended to passivate the stainless steel surface.
Chemical passivation as an optimal coating for heat-resistant steel
Metal passivation is a process as a result of which an oxide film is formed on the surface of the metal, preventing the formation of corrosion. The name of the coating method comes from the word “passivity”. The purpose of passivation is to reduce the chemical reactivity of a metal when interacting with other metals or aggressive environmental conditions.
In its own way, the appearance of a film is the same destruction of metal. But by destroying the top layer of material by several tens of nanometers, passivation saves the lower layers from rust.
Thus, chemical passivation is the interaction of an oxidizing agent with the surface being treated.
Stages of chemical passivation
1. If you do not prepare the metal product first, the oxidizing agent will react not with the alloy, but with foreign elements. Therefore, before passivation it is necessary to clean the surface . Cleaning is carried out in 2 ways: by washing or sanding the product using sandpaper. Now you can start passivation.
2. The process itself involves applying a chemical reagent to the product . A protective film is formed on the alloy, consisting mainly of salts and oxides. The film makes the structure of the product stronger and more durable. The effectiveness of the procedure depends on the following factors:
- composition of the solution;
- alloy composition;
- condition of the surface of the workpiece.
High-alloy steels, especially chromium-nickel steels, are best suited to chemical passivation. But carbon steels should be treated only for short-term protection, since the level of the protective layer on them is significantly weaker.
3. Cleaning with water . Any salts that may remain on the product may cause corrosion. Therefore, washing should be carried out carefully.
4. Residual acid must be neutralized with a 2-3% ammonia solution or a solution consisting of 25-30 g/l oleic acid and 2-4 g/l sodium hydroxide. Treatment is carried out at 80 – 90 °C for 2-3 minutes.
What solution is used?
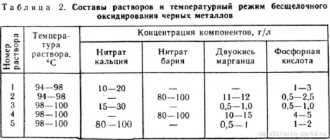
The use of different solutions depends on the properties of the alloy. Let's consider what solutions are used to passivate various classes of ferrous metals:
Highly alloyed alloys resistant to corrosion - nitric and sulfuric acids.
- Ferritic alloys - potassium dichromate, nitric acid.
- Carbon steels - potassium dichromate, chromic anhydride, phosphoric acid, sodium hydroxide.
- Medium alloy steels - chromic anhydride, phosphoric acid.
The passivation temperature and time also depend on the alloy class. The temperature ranges from 18 to 90 °C, and the time ranges from 3 to 60 minutes.
The higher the temperature of the solution, the faster the process proceeds.
Application of passivation
- Passivation is used for metal parts for painting. It not only protects against corrosion, but also degreases products. Used in the field of mechanical engineering.
- Passivation of steam turbines. But why do you need passivation of stainless steel, since it won’t rust anyway? It turns out that if the alloy is in continuous contact with an aggressive environment, it can collapse. An example is a weld. Sometimes there are iron particles on it. And then even stainless steel corrodes.
- Dental field. The lower part of the implants is processed - the screws that are mounted into the jaw. Passivation is used to prevent destruction of the implant in the jawbone.
- Chemical passivation is often carried out for decorative purposes. With short-term treatment, a rainbow film appears on the surface. Bright objects of use - taps, door handles.
- Passivation of costume jewelry is used to avoid allergic reactions.
Chemical passivation significantly extends the service life of metal products and deserves wide application in a wide variety of fields.
Passivation of surfaces
Almost all metals are quite durable materials. However, their structure and general condition can be affected by ordinary oxygen or liquid. Under the influence of an aggressive environment, plaque accumulates on the surface of metal products, which is corrosion. It is dangerous because under its influence the structure of the metal is destroyed, and the product made from it becomes unsuitable for further use.
Passivation has found widespread use in the modern world. It is not an easy procedure. It is almost impossible to cope with this without certain knowledge. The procedure is to dissolve the top part of the metal using an anode. In this case, the molecules break down into substances that have different levels of charge. In order for the ions to acquire an ordered appearance, an electric current is applied to the metal at a low voltage level, which is only 6-12 volts.
Ions are divided into positively charged and negatively charged. When an electric current passes through a metal, positively charged particles tend to the cathode, and negatively charged particles tend to the anode. It is at the anode that metal oxides are formed, which are the result of the splitting of the upper metal layer. As a result, a very thin protective film appears on the surface of the processed metal, which has unique protective qualities.
Passivation is aimed at making the metal less active. It becomes passive and is practically not affected by the environment.
In modern industries, this procedure is quite in demand. It helps protect metal surfaces from corrosion. The passivation process is used in situations where there is a need for careful preparation of the surface for applying paint and varnish. Also, this procedure is indispensable in those enterprises where metal objects often have to interact with an aggressive environment.
Passivation of metals is a useful procedure that renders these substances passive. It allows them to retain their properties for a long time. The thin film has an excellent level of protection, which gives metals additional strength and hardness.
Chemistry for pickling and passivation of steels
High-alloy steels are widely used in many sectors of the national economy, such as instrument making, food, chemical industries, aircraft shipbuilding, medical equipment production and many other areas due to a number of unique properties and characteristics of these materials. In the process of manufacturing parts and processing, steel is subjected to a huge number of both mechanical influences (cutting, welding, drilling, grinding) and chemical influences - treatment with reagents, contamination with oils, greases, dust, interaction with the wet palms of the operator.
During machining of a high-alloy steel workpiece, particles, sparks, small amounts of abraded metal from the cutting tool, and various oils and lubricants used in the metalworking process can penetrate the surface of the base metal. All these factors weaken the part’s resistance to corrosion and make it more susceptible to negative environmental factors. As a result, the top layer of the metal surface, the so-called passivation coating, is destroyed by these factors and corrosion can occur.
There are a huge variety of types of corrosion, in our article we will look at the main ones:
- Surface corrosion is determined by the destruction of metal over the entire surface in a uniform layer (Figure 1);
Figure 1 - Surface corrosion 1 - diagram of uniform surface corrosion; 2 - uniform surface corrosion on the part; 3 - local corrosion;
- Pit corrosion is accelerated local destruction of the metal surface in the form of shallow and wide ulcers (Figure 2);
Figure 2 - Pit corrosion
- Crevice corrosion – increased destruction of metal in cracks, crevices or gaps (Figure 3);
Figure 3 - Crevice corrosion
- Intracrystalline corrosion is a brittle fracture that is not accompanied by noticeable plastic deformation and occurs under the action of average stresses not exceeding the yield strength (Figure 4);
Figure 4 - Intracrystalline corrosion
- Stress corrosion is a special type of destruction of metals that occurs under the combined action of specific aggressive environments and tensile stresses (external and internal) (Figure 5).
Figure 5 - Stress Corrosion
Methods for processing metal products
To protect the metal surfaces being processed, surface treatment of high-alloy steels is carried out:
— Mechanical methods (grinding and sandblasting, shot blasting);
— Chemical methods (etching and passivation or electropolishing).
Mechanical methods. When grinding, the surface of the metal being processed is characterized by multiple small scratches, and when processing with abrasive particles, an electron microscope shows traces of balls on the surface after processing. Deformation of the metal surface after such types of processing is the main disadvantage of mechanical types of processing (Figure 6).
Figure 6 — Types of surface treatment of high-alloy steels
Chemical methods of surface treatment of high-alloy steels include etching and passivation. High alloy steels can be etched in four ways:
- applying with a brush the etching pastes MOST BLUE , ANTOX 71 E PLUS, ANTOX 71 E EXTRA, A NTOX 3 D ;
- application of pickling jelly liquids ANTOX 73 E , ANTOX 73 E EXTRA ;
- by immersing the part in a special liquid ANTOX 80 E;
- electrochemical.
Passivation is an effective method of restoring the corrosion resistance of stainless steel parts and components. The process cleans and improves the surface of parts, increasing their service life and creating a protective coating against environmental factors such as water, air and various contaminants.
Figure 7 shows schematically the structure of high-alloy steel before etching, then what happens to it during the process and what the structure of the etched and passivated steel looks like.
Figure 7 - Structure of high-alloy steel schematically
In Figure 8 you can see the structure of processed steel under a microscope.
Figure 8 - Microphotograph of steel
Applying etching passivating pastes with a brush
The manual method of applying etching and passivation paste by brush is used to remove scale and tarnish from welds obtained when welding stainless steels and nickel alloys, as well as for local processing of small areas of parts in hard-to-reach areas.
1. Degreasing Before carrying out the etching procedure, it is necessary to prepare the metal workpiece. To do this, use ACLEAN 400 to degrease the surface or ACLEAN 118 to degrease and remove light rust. The exposure time of these products is about 5-10 minutes.
2. Rinse with water Rinse with water under pressure
3. Etching and passivation. Then rinse, and then etching with special pastes MOST BLUE , ANTOX 71 E Plus , ANTOX 71 E Extra . The exposure time of the pastes is about 15-60 minutes (the desired temperature of use is 20oC, the permissible range of ambient temperatures is from min 10oC to max 30oC, the lower the ambient temperature limit when using chemistry, the higher the etching time.). Next, we carry out neutralization using M OST NEUTRALIZATOR and ANTOX NP , which are applied directly to the etching pastes MOST BLUE, ANTOX 71E Plus and ANTOX 71E Extra (Figure 9), for about 5 minutes.
4. Rinse with water At the end, it is recommended to rinse with water under high pressure of at least 140 bar using a plastic brush on the etched surface.
Figure 9 - Manual etching method - applying pastes with a brush
Application of pickling liquids and passivation by pump
To process large-sized parts or when it is not possible to use special baths, etching liquids are applied using special pumps ( manual or high pressure - Figure 10).
Figure 10 — Application of etching liquids and passivation with a pump
The application algorithm consists of the following stages:
1. Degreasing Perform degreasing with ACLEAN 400 . If it is necessary to degrease and remove light rust, then use ACLEAN 118 . To remove rust and brighten the surface of steel, you can use ANTOX 75 E. Exposure time: 5-20 minutes.
2. Rinse with water Rinse with water under pressure
3. Etching Then perform etching using ANTOX 73 E SG, ANTOX 73E EXTRA - over the entire etched surface. Action time: 20-60 minutes (at a temperature of 20oC). For better visibility, you can add dye
4. Rinse with water. Rinse with water under high pressure - at least 140 bar.
5. Passivation Carry out passivation using a solution of ANTOX 90E with water (1:1 ratio), applying to the entire etched surface. Action time: 20-30 minutes
6. Rinse with water. It is recommended to rinse with water under high pressure - at least 140 bar with demineralized water (without chlorides and sulfides).
Passivation by immersion
Passivation by immersion in a special bath is carried out in cases where it is necessary to process the entire part at once (Figure 11).
Figure 11 — Passivation by immersion method
1. Degreasing Just as in the manual etching method, before performing the etching procedure, it is necessary to prepare the metal workpiece. To do this, you need to degrease the surface using ACLEAN 400 or; if it is necessary to degrease and remove light rust, then ACLEAN 118 . To remove rust and brighten the steel surface we use ANTOX 75 E (Figure 12). The exposure time for these substances ranges from 5 to 20 minutes.
Figure 12 - Cleaning, brightening, removing foreign corrosion and rust using ANTOX 75 E
2. Rinse with water Rinse with water under pressure
3. Etching After rinsing, you can begin etching in the bath using ANTOX 80 E (mixed with water in a 1:1 ratio) for 20-60 minutes. The ambient temperature range should be from min 10oC to max 30oC. The lower the ambient temperature limit when using chemicals for etching stainless steel, the longer the etching time.
4. Rinse with water We rinse with water under high pressure - at least 140 bar
5. Passivation Then passivation with a concentrated solution of ANTOX 90 E (mixed with water in a 1:1 ratio). Exposure time: 20-30 minutes at a temperature of 10-30oC.
6. Rinse with water We complete the procedure by rinsing with water under high pressure from 140 bar.
It is recommended to use demineralized water (free from chlorides and sulfides).
Polishing and etching paste Antox 2001 T
Antox 2001 T special paste is intended for etching and removing tarnish from surfaces of welds made during TIG welding (argon arc welding), discoloration, as well as for cleaning heavily soiled or rusty equipment and products made of stainless steel (Figure 13).
Figure 13 - Removing tarnish when TIG welding polished surfaces
Particularly recommended for polished steel because it is the only pickling paste that does not leave the surface dull after use. Not recommended for external use. The delivery set includes an application and polishing kit (KIT A and KIT B), including cleaning products, a plastic spatula for applying the paste, polishing felt and gloves (Figure 14).
Figure 14 — Composition of the Antox 2001 T polishing kit
Method of application (Figure 15):
- Apply a small amount of Antox 2001 T polishing and etching paste to a piece of rag.
- Distribute evenly over the surface to be treated.
- Leave for 1-2 minutes.
- Wipe the surface to be treated with a rag.
- Wash off excess paste with water.
Figure 15 — Method of using Antox 2001 T paste
Etching time:
- rust, steel: 10-15 minutes;
- polished steel: 1-3 minutes.
Application method: manual.
Electrochemical etching
Another method of etching and passivation of stainless steel is electrochemical. The MOST RAPID pickling installation is used to remove the oxide film, remove dullness and tarnish from the surfaces of welds made during TIG argon arc welding. During processing, the part undergoes passivation, resulting in a layer that protects the part from corrosion. At the same time, the surface is polished (Figure 16).
Figure 16 — Electrochemical polishing process
The advantage of this processing method is that the process is significantly cheaper compared to cleaning and acid treatment. Chemical liquids used in the installation do not contain hazardous substances and do not require special labeling. Other advantages also include the ability to process parts of various geometric shapes, including in hard-to-reach places, and giving the part a status appearance. When electropolishing, the minimum possible level of roughness is achieved (Figure 17).
Figure 17 - Stainless steel surface before and after electrochemical polishing
The disadvantages of the electrochemical polishing process include the need for mechanical surface treatment before electropolishing; lack of a universal electrolyte for various metals and, as a result, its frequent change; the presence of a source of electricity as such and increased energy consumption; limited variety of processed materials, high technology.
Environmental protection and safety during pickling and passivation
Etching products MOST and Antox, as well as water after washing them off, cannot be poured into the sewer without special treatment. The water after washing off the chemicals for etching contains various acids, as well as elements of the metal being processed: iron, chromium, nickel. Neutralization should be done with calcium hydroxide. Neutralized water must be filtered. Sludge and sludge must be treated by an authorized organization.
Personnel working in the etching process are required to use acid-resistant clothing and personal protective equipment - goggles, rubber gloves, gas masks, etc. (Figure 18).
If etching solutions and pastes come into contact with your skin, you should immediately wash them off with water for 10 minutes and consult a doctor. Personnel working in the pickling process must undergo a medical examination at least once a year.
Figure 18 - Safety precautions for etching and passivation
All types of chemicals for pickling and passivation of high-alloy steels are always available in the warehouse at the address: Minsk, Lipkovsky Lane 30.
Cost and quantity, as well as DISCOUNTS, can be checked with managers by phone:
Tel.
/ Fax: +375 (17) 336-20-50 Mobile. tel. (MTS / A1): 572-20-20
The request can be sent by email: [email protected] or using the feedback form on the website.
Working hours:
Mon-Thu. 8.15-17.00
Fri. 8.15-15.45 Sat-Sun – closed
Follow us on social networks: Instagram Facebook YouTube
Properties of metal after processing
The main objective of passivation is to improve the physicochemical and mechanical characteristics of the surface layer of the material from which the part is made. The remaining characteristics of the deeper layers remain unchanged. Therefore, after passivation is completed, the following properties and characteristics change in the surface layer:
- a layer with a new chemical composition appears;
- anti-corrosion activity changes (it slows down significantly);
- the physical characteristics of the material are improved (only the surface layer);
- in some cases, the mechanical strength of the product increases;
- the color of the part changes (it takes on a more aesthetic shape);
- consumer properties increase and presentation improves.
Passivation of stainless steel can significantly improve anti-corrosion properties and give the finished part a completely different color. The use of chromium or nickel in the passivation solution produces a shiny metallic color.
Passivation of iron with chemical elements close to it makes it possible to create an outer layer that is sufficiently resistant to corrosion. In this way, the scope of application of such products expands. They can be used even in active and aggressive environments. In addition to various grades of steel, cast iron is subjected to passivation. The main task is to create a protective film against corrosion. In some cases, when thickened sodium nitrate is used, the surface layer acquires some elasticity. In this case, the fragility of the entire part is reduced. One type of steel is the so-called blued finish. The result of processing is a reliable outer layer of black color.
The properties of the surface layer of non-ferrous metals change in a similar way. As a result of passivation, adsorption or phase layers of a certain thickness are formed. Placing an aluminum workpiece stimulates the process of natural passivation of the surface layer of this metal. When exposed to acidic solutions, the protective properties of the surface layer of aluminum increase.
Principles of cleansing at home
Tips for caring for stainless steel are very simple. They are easy to follow.
What not to use
List of products and devices that should not be used when using stainless steel cookware:
- Dishwasher;
- metal sponge;
- a cleaner containing abrasive components.
How to use baking soda and salt
Salt and soda are essential care products for stainless steel cookware. The principle of their use is simple:
- the product is washed;
- apply soda, salt or a mixture thereof to the contaminated area;
- rub the powder in a circular motion.
After cleaning, the item is rinsed with water and dried with a towel.
Timeliness
Regular cleaning of stainless steel pans eliminates the appearance of old stains. It takes little time to remove fresh stains.
Application of passivation
The main objectives of passivation include:
- prevention of corrosion processes occurring in the upper layers of metal;
- protection from destruction of newly created connections, for example, at the site of a weld (passivation of welds);
- increased electrical conductivity at the point of electrical contact;
- creation of printed circuit boards using prepared templates (etching);
- processing of the finished product in order to impart new decorative and consumer properties.
The first problem is solved for a large number of metals and their alloys. One option for such protection is bluing. In the second case, to create a strong welded joint, passivation of the anodes and final processing of the resulting joint after welding are used. Carrying out passivation can significantly increase the tightness of the resulting connections. This is especially important when laying pipelines. This treatment is very useful when welding difficult-to-weld metals such as aluminum. Passivation of copper or brass is carried out to create temporary protection against tarnishing of the surface of the product for a certain period of time (usually about a month). Sometimes it is used as a temporary preservation of prepared parts for storage between further processing or assembly operations.
This type of processing is necessary when using metal products in the following cases:
- the use of fasteners, especially in aggressive environments and high mechanical loads;
- when assembling pipelines, especially in places of welds;
- to protect boiler equipment;
- machine parts and mechanisms in contact with sea water;
- structural elements operating under changing temperature conditions;
- individual elements of hand and mechanical tools;
- finished products used in everyday life (door handles, furniture fittings, etc.);
- decorative crafts for the interior;
- in radio electronics to improve the quality of contacts;
- Jewelry.
The problem of increasing electrical conductivity is solved by applying a thin layer of metal with increased electrical conductivity, such as gold or silver, to the surface of the manufactured contacts.