Physics is an incredibly fascinating science, once you figure out what's what. And the formulas in it reflect real physical processes, only in numbers. And if you understand why the formula is the way it is, then it will be much easier to learn. But it’s impossible to tell everything at once, and today we’ll figure out how to find mass through density and volume.
Before you begin to study the formulas for mass, density and volume, you should clarify some details:
- Firstly, the volume of a substance depends on temperature . When heated, a solid expands, and at low temperatures it contracts. There are also special issues, as in the case of liquid hydrogen. It cannot exist at high temperatures because it will turn into gas.
- Secondly, different organizations and countries have their own standards for the conditions under which measurements are taken . In other words, the numerical density of the same substance will differ in different countries. Therefore, before asserting that the indicators are incorrect or correct, it is necessary to clarify the conditions under which these indicators were obtained.
- Thirdly, in addition to temperature, the volume factor can also be influenced by indicators such as atmospheric pressure . It is especially important when measuring the density of gases, since it has practically no effect on solids.
The formula and the amazing history of its origin
The most common formula for most cases is: m = pV, where m is the mass of the body, p and V are the density of the substance and its volume occupied in space, respectively. You can, of course, not bother and calculate everything on online resources, but knowing the formula is still useful. Accordingly, V = m/p, p = m/V.
The most interesting thing is that the formula was found by a man who ran naked down the street and was at the same time a friend of the king. Interesting? Then the next three paragraphs are for you.
There was such a tyrant king in Ancient Greece as Hiero II. He began to suspect that his crown was not made of pure gold and that the jewelers had cheated him. But Hiero did not know how to prove this. Then he turned to the smartest man of that time - Archimedes. Having received an order to deal with matters of national importance, Archimedes day after day began to look for a solution to the issue.
Oh, and the scientist had a difficult task. After all, at that time there were neither the necessary formulas , nor modern devices, nor Google to quickly find a solution. And then one day, having come to the bathhouse and immersed himself in it, Archimedes noticed that the pouring water was equal in volume to what was immersed in the water.
Eureka! – Archimedes shouted and hurried naked to his laboratory to conduct experiments. The scientist put all the data in his head and later did the following experiment: he took the crown and lowered it into the water. Then he took a piece of gold of the same weight and lowered it into the water. The volume of displaced water turned out to be different. If the crown were made of pure gold, then its volume and the volume of the ingot would match. This proved that the jewelers had deceived the king. Who would have thought that one of the greatest discoveries came about thanks to deceivers, a tyrant and a scientist.
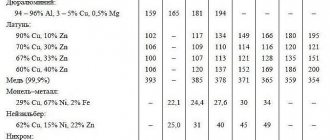
Table of densities of some substances
The density of many substances is known in advance and can be easily found using the corresponding table.
When working with it, it is important to pay attention to the dimensions and not to forget that all data was collected under normal conditions: room temperature of 20 degrees Celsius, as well as a certain pressure, air humidity, and so on.
Densities of other, rarer substances can be found online.
At least one of the density values is worth remembering, as it often appears in problems. This is the density of water - 1000 kg/m3 or 1 g/cm3.
Designations and terms
The following is a list of concepts and their definition in terms of concepts about density measurements:
- Mass is the density of a body multiplied by its volume occupied in space. This is also a quantity that determines the strength of the gravitational field on an object.
- Volume is a physical quantity that characterizes the amount of space occupied by an object.
- Density determines how much of a substance fits in a volume at a certain weight under standard conditions.
- Normal/standard conditions have different meanings in different organizations. These conditions include ambient temperature, atmospheric pressure and, in some cases, other parameters.
- Atmospheric pressure is a concept used more for gases, since it has a greater influence on their volume than on solids. Atmospheric pressure can be defined as the force exerted by the air on the Earth under the influence of the gravitational field.
- Temperature is a physical indicator of the degree of heating of a substance. The higher the temperature, the greater the volume of the body.
Peculiarities
In the case of porous or granular substances (the former includes, for example, shell rock, the latter – cereals from which porridge is prepared), two densities are distinguished:
- true - minus voids filled with air or liquid;
- bulk - determined by the method described above, when the volume of porous/bulk material includes voids.
The real density is calculated from the apparent (bulk) density through a coefficient determined in practice - excluding voids.
As temperature increases, the density of a substance decreases, although there are exceptions, for example, water. At 4 °C it is most dense; with cooling and heating, the value decreases, and ice is lighter than liquid water.
Examples of problem solving
Before proceeding with the examples, it should be understood that if the data is given in kilograms and cubic centimeters, then you need to either convert centimeters to meters, or convert kilograms to grams. Using the same principle, it is necessary to translate the remaining data - millimeters, tons, and so on.
Task 1 . Find the mass of a body consisting of a substance whose density is 2350 kg/m³ and has a volume of 20 m³. We apply the standard formula and easily find the value. m = p*V= 2,350 * 20 = 47,000 kg.
Task 2 . It is already known that the density of pure gold without impurities is 19.32 g/cm³. Find the mass of a precious gold chain if the volume is 3.7 cm³. Let's use the formula and substitute the values. p = m / V = 19.32/3.7 = 5.22162162 g.
Task 3 . Metal with a density of 9250 kg/m³ was delivered to the warehouse. Weight is 1,420 tons. We need to find the volume occupied by the metal. Here you first need to convert either tons to kilograms, or meters to kilometers. It will be easier to use the first method. V = m/p = 1420/9250 = 0.153513514 m³.