Oxidation
Oxidation is the process of forming oxide films on the surface of a metal. Oxidation is used to apply oxide layers, both for protection purposes and to impart decorative properties to a metal product.
Metal oxidation can be carried out in several ways:
— chemical oxidation;
— thermal oxidation;
— anodic oxidation (electrochemical);
— flame methods (micro-arc oxidation, etc.).
Chemical oxidation
Chemical oxidation is carried out by treating the product in solutions (melts) of oxidizing agents (chromates, nitrates, etc.). Using this method, the surface of the product is passivated or by applying protective and decorative layers. For ferrous metals, chemical oxidation is carried out at temperatures from 30 to 100 °C in alkaline or acidic compositions.
For acid oxidation, a mixture of several acids is mainly used, for example, nitric (or orthophosphoric) and hydrochloric acids with some additives (Ca(NO3)2, Mn compounds). Alkaline oxidation is carried out at slightly higher temperatures, about 30 – 180 °C. Oxidizing agents are added to the composition. After applying the oxide layer, the metal products are thoroughly washed and dried.
Sometimes the finished coating is oiled or further processed in oxidizing solutions.
Protective layers obtained using chemical oxidation have less protective properties than films obtained by anodization.
Thermal oxidation
Thermal oxidation is the process of formation of an oxide film on a metal at elevated temperatures and in oxygen-containing (maybe water vapor) atmospheres. Thermal oxidation is carried out in heating furnaces. During thermal oxidation of low-alloy steels or iron (an operation called bluing), the temperature is raised to 300 - 350 °C.
For alloy steels, thermal oxidation is carried out at higher temperatures (up to 700 °C). The duration of the process is about 60 minutes. Very often, thermal oxidation is used to create an oxide layer on the surface of silicon products. This process is carried out at high temperatures (800 – 1200 °C). Oxidized silicon products are used in electronics.
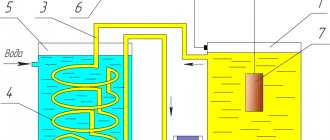
Anodizing (electrochemical or anodic oxidation)
Anodizing is one of the ways to obtain an oxide film. Anodizing is carried out in liquid or solid electrolytes. When anodizing, the surface of the metal that is oxidizing has a positive potential. Anodizing is used to obtain protective and decorative layers on the surfaces of various metals and alloys.
Anodizing is most often used to obtain a coating on aluminum and its alloys. On aluminum, layers with protective, insulating, wear-resistant, and decorative properties are obtained.
Plasma methods for deposition of oxide layers
Plasma oxidation is carried out at low temperatures in plasma, which contains oxygen. Plasma for this type of oxidation is formed using direct current discharges, microwave, and HF discharges.
Plasma oxidation is used to obtain oxide layers on various semiconductor compounds and silicon surfaces. Plasma oxidation can increase the photosensitivity of silver-cesium photocathodes.
Microarc oxidation
Microarc oxidation (MAO) is a method for producing multifunctional oxide layers. Micro-arc oxidation is a step up from anodizing. Allows you to apply layers with high protective, corrosion, heat-resistant, insulating, and decorative properties. In appearance, the coating obtained by the micro-arc method is very similar to ceramics.
Now this is one of the most promising and popular methods of applying oxide layers, because allows you to apply heavy-duty coatings with unique characteristics.
The microarc oxidation process is carried out, in most cases, in weakly alkaline electrolytes when applying pulsed or alternating current. No special surface preparation is required before applying the coating. The peculiarity of the process is that...
That energy is used from electrical microdischarges that move chaotically across the surface being treated. These microdischarges have a plasma-chemical and thermal effect on the coating and electrolyte. The oxide layer is approximately 70% formed deep into the base metal.
Only 30% of the coating is completely outside the product.
The thickness of coatings produced by the micro-arc method is about 200 – 250 microns (quite thick). The electrolyte temperature can range from 15 to 400 °C, and this does not have much effect on the process.
The electrolytes used do not have a harmful effect on the environment and their service life is very long. The equipment is compact, does not take up much space and is easy to use.
The dissipative ability of the electrolytes used is high, which makes it possible to obtain coatings even on parts with complex relief.
Microarc oxidation is used to form coatings mainly on magnesium and aluminum alloys.
What is the oxidation method?
Most metallic substances enter into an active phase with various chemicals. In some cases, it occurs with the release of a third-party substance, which can become protection for the main product. In the method under consideration, an oxide film appears after applying a special solution to the surface.
The liquid, under the influence of the redox reaction, leads to the creation of a top layer that increases corrosion resistance and also decorates the plane. It should be noted that there are several varieties of the process; they are selected depending on what effect needs to be achieved, as well as what material is being processed. Let's take a closer look at the views.
Aluminum oxidation (anodizing, chemical oxidation)
Products made of aluminum and its alloys are subjected to special treatment to increase corrosion resistance - oxidation using both chemical and electrochemical methods (aluminum anodization).
Oxidation not only increases the corrosion resistance of products made of aluminum and its alloys, but also has a beneficial effect on the performance characteristics of the processed parts, increasing their hardness and wear resistance, heat resistance and heat resistance, giving them a variety of electrical properties, etc.
The thermal emissivity coefficient of oxidized aluminum reaches 80% of its value for a completely black body.
The oxide layer in most cases has a microporous structure and, as a result, has a high adsorption capacity, which can affect both positively and negatively the quality of the coating.
To obtain high-quality oxide coatings, a rigid, springy contact of the workpiece with the hanging device is required. Pendants for oxidation are made of aluminum, duralumin or titanium.
Our organization offers services in Kyiv for anodizing and chemical oxidation of aluminum in colors such as black, colorless, light green and pale orange.
Chemical oxidation of aluminum
Chemical oxidation of aluminum and its alloys is carried out to protect products from corrosion or as a primer for painting. The thickness of the oxide films is 0.5-4.0 microns.
Films obtained by chemical oxidation are significantly inferior in their protective and physical-mechanical properties to those obtained by electrochemical oxidation of aluminum.
Chemical oxidation is used mainly in cases where the anodizing process is difficult and economically impractical when coating complex and large-sized parts, internal surfaces of long and thin-walled pipes, large welded structures, as well as for oxidizing parts of minor purposes. It should be taken into account that the chemical oxidation process is very simple to operate and is more economical than electrochemical oxidation.
Chemical oxidation of aluminum and its alloys is carried out in alkali-chromate, phosphate-chromate and chromate-fluoride solutions.
Alkali-chromate solutions form oxide films 2 microns thick with low mechanical properties. These films are used mainly as a primer for painting.
Phosphate-chromate solutions form oxide films of greater thickness up to 3-4 microns with higher protective and physical-mechanical properties. These coatings are used to protect products from corrosion, and also as a primer for paintwork.
Films made from chromate-fluoride solutions are thin but dense and have low electrical resistance. They are used to produce conductive oxide coatings. Color – orange with a brownish-reddish tint. This coating is called conductive or fluoride.
Electrochemical oxidation (anodizing aluminum)
Electrochemical oxidation of aluminum and its alloys is one of the most common processes in modern galvanic production. This process is called anodization. Anodizing allows you to widely change surface properties, such as corrosion resistance, hardness, wear resistance, and electrical properties.
During anodic oxidation, two processes occur simultaneously: the formation of an oxide film on the anode and its dissolution by anodizing electrolyte. If the resulting film does not dissolve in the electrolyte, then thin compact films are formed, practically non-porous with high electrical resistance, the growth of which stops when the entire anode is covered with a film.
To form thick anodic films, it is necessary to ensure access of oxygen ions to the anode surface during the entire electrolysis time. This occurs in electrolytes that have a certain dissolving effect on the oxide film.
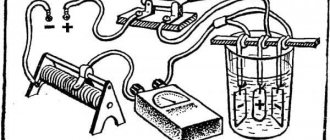
With your own hands
The methods presented above are used only in production, but if you are ready for independent experiments, then you need to create a small home laboratory.
For the experiment, take a small steel piece that will fit into a three-liter jar without any problems.
Stages of work
Do each of them consistently and thoroughly. Prepare all the necessary tools in advance.
Rough stripping
Use a steel brush or coarse sandpaper. You need to remove all rust down to the base, as well as other contaminants. It is better if you then go over it with fine-grained sandpaper to ensure a uniform surface.
Polishing
Special pastes with fine abrasives or discs on hand-held grinders are perfect.
Plaque removal
In other words, rid the element of grease, oil traces, and polishing paste residues.
Treatment
To do this, introduce a solution of sulfuric acid with a 5% content of the substance and place the workpiece there for 1 minute.
Washing
First, rinse the part in regular running water, and then boil it in a soapy water mixture. Now make a 5% solution of caustic soda in a container, place the workpiece there and heat to 150 degrees, hold for 2 hours. Then just let it cool and evaluate the result. You've got an oxidized coating - this is a wonderful effect achieved at home. To clarify the information you are interested in and purchase Russian-made metal bandsaw machines, contact the managers by phone 8 (908) 135-59-82;;. They will answer all your questions.