No material, including steel, can last forever. It must be protected from moisture, sunlight and low temperatures. Oxidation of a metal creates a thin protective film on its surface that prevents oxygen from the air and water from destroying the material. At the same time, the technical characteristics of steels, aluminum and its alloys change.
From a chemical point of view, oxidation is the oxidation reaction of a metal and the formation on the surface of a thin layer of crystals bound by oxygen and other substances. The technology for applying a protective coating has several types of varying complexity. The simplest one was used several centuries ago and is available to anyone who wants to cover a part with a protective film at home. Complex technology requires special equipment and is carried out only in production conditions.
What is the oxidation method?
Most metallic substances enter into an active phase with various chemicals. In some cases, it occurs with the release of a third-party substance, which can become protection for the main product. In the method under consideration, an oxide film appears after applying a special solution to the surface.
The liquid, under the influence of the redox reaction, leads to the creation of a top layer that increases corrosion resistance and also decorates the plane. It should be noted that there are several varieties of the process; they are selected depending on what effect needs to be achieved, as well as what material is being processed. Let's take a closer look at the views.
Aluminum
How to clean stainless steel: contamination of stainless steel and methods for cleaning it (video 125 photos)
Anodized coating is performed to increase corrosion resistance and prepare for painting. And also, depending on the technology used - either to increase roughness or to create a smooth surface. At the same time, anodizing in itself is not capable of significantly increasing the strength of products made from this metal. When aluminum comes into contact with air or any other gas containing oxygen, the metal naturally forms an oxide layer 2-3 nm thick on its surface, and on alloys its value reaches 5-15 nm.
The thickness of the anodized aluminum coating is 15-20 microns, that is, a difference of two orders of magnitude (1 micron is equal to 1000 nm). Moreover, this created layer is distributed in equal parts, relatively speaking, inside and outside the surface, that is, it increases the thickness of the part by ½ the size of the protective layer. Although anodizing produces a dense and uniform coating, microscopic cracks in the coating can lead to corrosion. In addition, the surface protective layer itself is subject to chemical decomposition due to exposure to an environment with high acidity levels. To combat this phenomenon, technologies are used that reduce the number of microcracks and introduce more stable chemical elements into the oxide composition.
Methods
Each of them has a certain popularity, most are used in factories under certain conditions. But there is the possibility of doing metalworking yourself. At the same time, it is worth remembering the possible negative effects on the body and means of protection.
Chemical oxidation of steel, technology
A liquid solution, dry mixture or melt is applied to the surface of a metal product. Then a reaction occurs between these elements (under certain conditions, for example, with access to oxygen, at a set temperature). Its result is the formation of an inactive top layer - this procedure is called passivation, that is, the top layer is made passive in relation to certain environments. Chromium oxides are most often used for these purposes.
The workpiece is fixed in one way or another and immersed in a bath with the prepared solution (this is possible under several conditions - with the appropriate dimensions of the object and the tank and with the walls of the vessel that do not react). The alkaline or acidic composition is created in advance and has a certain percentage. Depending on the parameters, the degree of exposure is determined. After the required time, the part is taken out, dried, and then finishing metalworking work is carried out. When creating an acid bath, acids such as hydrochloric, nitric, or phosphoric are most likely used. If you add capsules of manganese, chromium or potassium, the flow will be accelerated. Usually the temperature range is selected from +30 to +100 degrees. If the base is a combination of sodium nitrate and manganese dioxide, then we can talk about using an alkaline solution, which heats up much more strongly - up to 300 degrees. There are also two varieties when additional substances are used that affect the quality of the result obtained:
- Potassium dichromate allows you to consolidate the final achievements;
- oil - this process is called oxidation with oiling or chemical oxidation, in which it is possible not only to achieve high resistance to rust, but also to obtain a black glossy surface color.
Thermal oxidation
This is a similar method in which a protective oxide film is formed, but it takes place at elevated temperatures and in direct contact with water vapor or oxygen. This action requires special furnaces that can maintain operating conditions up to 1200 degrees - different materials have their own characteristics.
If you want to improve the effect, we recommend first immersing the part in a soap solution for a few minutes, and then drying it and filling the bath with machine or transformer oil. If you heat to 105 degrees or higher, you can achieve a uniform, shiny black surface.
Anodic oxidation - what is it?
It is also called electrochemical oxidation or anodization. This is another option for quickly obtaining an oxide film in a liquid or dry mixture. The main process that underlies the technology is electrolysis, which, as is known, can take place in both liquid and solid media.
The element is placed in solution. A potential difference is formed between it and the liquid - in the upper layers it is initially positive, and in the mixture it is negative. It should be noted that the application of voltage, as well as the use of active reagents, leads to the fact that the procedure is considered unsafe, at least for home implementation. When anodizing, two goals are achieved - decorative design and the creation of a protective layer. Most often, this is subjected to aluminum, which by its nature does not have sufficient characteristics of rigidity, strength, and resistance to mechanical stress. Depending on what acid is used, as well as what voltage parameters are set, the resulting film can be of varying thickness. They will be thin if you use B(OH)₃ (boric) or H₃PO₄ (orthophosphoric). But if you need to give an interesting shade to oxidized steel, then you should use organic acids, for example, oxalic, maleic, sulfosalicylic. Weakly alkaline compounds are also used to immerse parts in them and pass a weak alternating or pulsed current. This process is called micro-arc machining and is distinguished by the fact that good results can be achieved. The surface not only looks good and does not rust, but also becomes more heat-resistant and acquires insulating qualities.
A special approach must be applied to stainless steel. It is an inert, that is, neutral alloy. As you can understand, achieving a potential difference in this case is quite difficult. Therefore, the procedure becomes two-step. First, double anodization is carried out - that is, not only stainless steel is immersed in a bath with the composition, but also another element, which, according to its characteristics, is more suitable for the process. Nickel and copper are suitable for these purposes.
The second stage is the independent oxidation of stainless steel. But to increase efficiency and speed up the achievement of results, it is recommended to apply passivating pastes. Their purpose is to speed up the reaction.
Plasma method
It is also called micro-arc. Its peculiarity is that it creates plasma with a high oxygen content. At the same time, it does not heat up and maintains low temperatures. The flow generation itself occurs under the influence of charges, which, in turn, are formed under the influence of alternating or pulsed current of high or ultrahigh frequency. Typically, the method is used when it is necessary to create an oxide film for protection on a relatively small surface of the product. The technology is most often used in electronics and microelectronics, for example, in the production of semiconductors, transistors, diodes, and microcircuits. The second purpose is to increase photosensitivity, so the procedure is used to increase sensitivity in photocathodes. Sometimes it is still more advisable to make plasma at a higher temperature - up to 430 degrees and above. The quality is greatly improved. The advantages of microarc oxidation include:
- The oxide layer can reach up to 70% deep into the main workpiece.
- Thickness is about 200 – 250 microns.
- It is very good to process elements with complex terrain.
- Excellent behavior on magnesium and aluminum alloys.
Laser
In order to increase corrosion resistance, it is possible to achieve the formation of an oxide film on steel using a laser installation. The peculiarity of the process is that to carry it out you definitely need a specialized machine. The use of a fiber laser in the infrared range has proven to be most effective. Three methods can be used:
- pulsed radiation;
- continuous luminous flux;
- spot heating of the material.
Note that the technology requires quite high costs and is also not suitable for large metal structural elements. Good for limited planes using CNC machines.
DIY oxidation
If you need to make an oxide film at home, then for a good result you should strictly follow the sequence of actions, as well as adhere to safety rules.
Below we will talk in more detail about the step-by-step implementation of independent oxidation, but first we will discuss why do this?
Technological parameters of sulfate anodization
Sulfuric acid electrolyte
Sulfuric acid-based electrolytes are commonly used to anodize extruded aluminum profiles throughout the world.
Qualanod sets the following parameters for the sulfuric acid electrolyte:
- The concentration of free sulfuric acid should be no higher than 200 g/l with fluctuations within the range of 10 g/l from the specified value;
- The aluminum concentration should not exceed 20 g/l, preferably in the range from 5 to 15 g/l.
Anodizing bath temperature
Qualanod instructions for anodizing bath temperature:
- for a given anodic layer thickness of 5 µm and 10 µm: not higher than 21 ºС
- for a given thickness of the anode layer of thickness 15 microns, 20 microns and 25 microns: not higher than 20 ºС.
Current Density
Qualanod recommends an average current density:
• 1.2 – 2.0 A/dm² for anode coating with a thickness of 5 µm and 10 µm • 1.4 – 2.0 A/dm² for an anodic coating with a thickness of 15 µm • 1.5 – 2.0 A/dm² for anode coatings with a thickness of 20 microns • 1.5 – 3.0 A/dm² for an anodic coating with a thickness of 25 microns.
Aluminum alloys for anodized profiles
For aluminum profiles that will be anodized, alloys 6060 and 6063 are usually used with some restrictions on the content of magnesium and silicon, as well as impurity elements such as iron, copper and zinc.
Typically, the purer the aluminum and the fewer alloying elements it contains, the better it is anodized. An increased content of impurities in the alloy leads to the formation of inclusions in the anodic coating, which adversely affect the uniformity of its appearance.
See the influence of the chemical composition of aluminum alloys on the quality of anodized profiles here.
Changing the thickness of the anodic coating during anodizing
The thickness of the finished anodic coating depends on the total duration of anodizing. However, the rate of growth of the coating thickness depends on several factors, such as the composition of the electrolyte, the current density and the current duration of treatment.
During anodization, two competing processes occur (Figure 4):
- continuous increase in the thickness of the anodic coating and
- dissolution of the anodic coating under the influence of electrolyte.
Figure 4 – Change in coating thickness during anodizing
The theoretical value of the coating thickness at a constant current density obeys the well-known Faraday law. From this law it follows that aluminum oxide grows in proportion to the amount of electricity that passes through the anode (aluminum profile).
Effect of electrolyte temperature
An increase in the temperature of the electrolyte leads to a proportional increase in the rate of dissolution of the resulting anodic coating. As a result, the anodic coating becomes thinner, more porous and softer.
Effect of current density
The current density range that is used in standard anodizing is from 1 to 2 A/dm2 and in some cases up to 3 A/dm2. Current densities below 1 A/dm2 produce soft, porous and thin coatings. With increasing current density, the anodic coating is formed faster and with relatively less dissolution by the electrolyte. Therefore, the coating is harder and less porous.
Effect of sulfuric acid concentration
The effect of an increased concentration of sulfuric acid on the formation of an anodic coating is similar to an increase in temperature, although the effect of temperature is more significant. A high concentration of sulfuric acid may limit the ability to obtain a thick anodic coating due to the increased ability of the electrolyte to dissolve the forming porous aluminum oxide.
What does the process provide?
Manufacturers of metal parts know that the main problem why their products quickly fail is the formation of corrosion. The fact is that virtually any substance with metallic properties is quite strongly influenced by the external environment. These are humidity, temperature changes, solar radiation, reactions with oxygen, as well as pollution and natural wear and tear. Let's see what oxidation does for manufacturers.
Anti-corrosion properties
Even with constant exposure to the street in the rain and contact with air, rusting does not occur. This is very important for car body elements and other items that are primarily used outdoors.
Limiting environmental exposure
There are some products that are aggressors to metal. Simply put, they destroy its surface and even penetrate deeper into the structure, violating its integrity. These are vapors of chemicals or liquids, as well as ordinary ultraviolet radiation.
Electrical insulation characteristics
A number of parts must become dielectric, that is, not allow electricity to pass through. The created dielectric layer copes with this task perfectly.
Giving an original decorative look
It can be a black glossy sheen or a more exotic shimmer of various colors. It looks very nice, and practicality remains just as high.
Metal protective coatings
Anodic metal coatings are metals whose electrochemical potential is lower than that of the materials being processed. On the contrary, it is higher for cathode ones.
Cathodic coatings prevent the penetration of aggressive media into the base metal due to the formation of a mechanical barrier. They better protect surfaces from negative influences, but only if they are undamaged.
Depending on the method of application, metal coatings are divided into the following types.
Electroplating
Galvanization is an electrochemical method of applying a metal protective coating to protect surfaces from corrosion and oxidation, improve their strength and wear resistance, and give an aesthetic appearance.
Galvanic coatings are used in aircraft and mechanical engineering, radio engineering, electronics, and construction.
Depending on the purpose of specific parts, protective, protective-decorative and special galvanic coatings are applied to them.
Protective ones are used to isolate metal parts from aggressive environments and prevent mechanical damage. Protective and decorative are designed to give parts an aesthetic appearance and protect them from destructive external influences.
Special galvanic coatings improve the characteristics of the treated surfaces, increasing their strength, wear resistance, electrical insulating properties, etc.
Thermal spraying
It represents the transfer of molten particles of material to the surface being treated by a gas or plasma stream. Coatings formed by this method are distinguished by heat and wear resistance, good anti-corrosion, anti-friction and extreme pressure properties, electrical insulating or electrical conductivity. The materials to be sprayed are wires, cords, powders made of metals, ceramics and metal-ceramics.
The following methods of thermal spraying are distinguished:
- Flame spraying: the simplest and most inexpensive method used to protect large surface areas from corrosion and restore the geometry of parts
- High-speed flame spraying: used to form dense cermet and metal coatings
- Detonation spraying: used for applying protective coatings and restoring small damaged surface areas
- Plasma spraying: used to create refractory ceramic coatings
- Electric arc metallization: for applying anti-corrosion metal coatings to large surface areas
- Spraying with fusion: used when there is no risk of parts deformation or it is justified
Immersion in melt
When using this method, the workpieces are dipped into molten metal (tin, zinc, aluminum, lead). Before immersion, the surfaces are treated with a mixture of ammonium chloride (52-56%), glycerin (5-6%) and chloride of the metal being coated. This allows you to protect the melt from oxidation, as well as remove oxide and salt films.
This method cannot be called economical, since the applied metal is consumed in large quantities. At the same time, the thickness of the coating is uneven, and it is not possible to apply the melt into narrow gaps and holes, for example, on threads.
Thermal diffusion coating
This coating, the material for which is zinc, provides high electrochemical protection of steel and ferrous metals. It has high adhesion, resistance to corrosion, mechanical stress and deformation.
The thermal diffusion coating layer has the same thickness even on parts of complex shapes and does not peel off during operation.
Cladding
The method is the application of metal using a thermomechanical method: by plastic deformation and strong compression. Most often, protective, contact or decorative coatings are created in this way on parts made of steel, aluminum, copper and their alloys.
Cladding is carried out through the process of hot rolling, pressing, extrusion, stamping or explosion welding.
Chemical oxidation of steel: advantages
Now we list the features that can be achieved if you use the technology of creating an oxide film using chemicals.
Reliable anti-corrosion coating
The steel part actually becomes stainless steel.
That is, rusting, although not completely eliminated, is very significantly inhibited.
Good electrical insulators
After chemical treatment, one can expect that the surface completely or partially ceases to conduct current. Everything will depend on what solution was taken, in what concentration, etc.
Thin but durable surface layer
Interestingly, a film thickness of only 200 microns can be achieved. But this does not make it more susceptible to mechanical or other pests.
Original color scheme
This is more a sign of anodization.
But we note that after the procedure you can get not only black color, but also iridescent waves from yellow to dark blue, as in the photo.
Anodizing aluminum at a low price (anodic oxidation, aluminum oxidation)
Stainless steel railings: varieties and design
You can order anodizing services. aluminum in our campaign. We guarantee the quality of the applied galvanic coatings on products. To clarify the cost of anodizing, contact our manager.
What is anodizing (anodic oxidation, an.ox.)
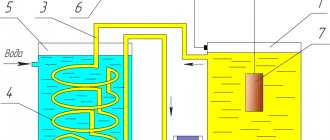
Anodization (oxidation) – electrochemical oxidation, the formation of a protective oxide film on the surface of metal products by electrolysis.
When anodizing, a product immersed in an electrolyte is connected to a positively charged electrode of a current source (anode).
A film with a thickness of 1 to 200 microns protects metal from corrosion, has electrical insulating properties and serves as a good basis for paint and varnish coatings.
Application of anodic oxidation of parts
Anodizing is used for decorative finishing of products made of aluminum and its alloys, enamel-like coatings on aluminum and some of its alloys, and is also used to protect magnesium alloys from corrosion, increase the antifriction properties of titanium alloys, and to coat electronic equipment parts made of niobium, tantalum, etc., in aircraft, rocket and instrument engineering, radio electronics.
Immediately after machining, aluminum reacts with oxygen in the air, so under normal conditions the surface is always covered with a thin oxide film. The structure of the film and its composition depend on the influence of atmospheric phenomena.
But aluminum always has an oxide film 2-3 nm thick. This film protects the metal from further oxidation and has excellent electrical conductivity.
The oxide film forms on pure aluminum at room temperature and has an amorphous structure (not crystalline) and is therefore not a good corrosion protection.
Aluminum protective coating
Aluminum is protected from corrosion by creating a crystalline oxide film 20-30 microns thick on its surface. In subsequent stages of the anodizing process, this film may be colored or may retain its natural color.
Anodizing aluminum also allows you to obtain various decorative effects, such as a mirror surface, matte and semi-matte surface, imitation of polished and ground stainless steel.
Aluminum anodizing process
Before starting the anodizing process, it is necessary to clean the aluminum surface from contaminants and remove the oxide film. To do this, degreasing and etching processes are carried out.
A process that results in the formation of highly porous aluminum oxide layers on the metal surface. The anodizing process is electrochemical.
There are two types of oxide films that are formed during the anodizing process:
Barrier - oxide film grows in neutral solutions in which aluminum oxide is difficult to dissolve. These are mainly ammonium borates, phosphates or tartrates.
In the first seconds of anodization, a barrier layer forms on aluminum, first forming in the active centers on the surface of the metal. From these embryos, hemispherical lens-shaped microcells grow, which then grow together into a continuous barrier layer. When in contact with six surrounding cells, the shape of a hexagonal prism with a hemisphere at the base is formed.
Under the influence of local effects of electrolyte ions, pores appear in the barrier layer (in the center of the cells), the number of which is inversely proportional to the voltage.
In the pore, the thickness of the barrier layer decreases and, as a result, the electric field strength increases, while the ion current density increases along with the oxidation rate.
But, since the temperature in the pore channel also increases, which promotes etching of the pore, dynamic equilibrium sets in, and the thickness of the barrier layer remains practically unchanged.
This completes the anodizing process, and we obtain a coating with remarkable optical and technological properties.
Advantages of anodizing products
Anodized products can serve for decades without changing their decorative properties. Anodic corrosion protection is so effective that it can protect parts from the most aggressive influences. These remarkable properties have long been appreciated by car manufacturers, builders, military, and aircraft manufacturers.
Thermal oxidation
Let's present a table with some alloys that are most often subjected to oxidation:
| Name | Temperature, °С | Features, purpose, use |
| Low alloy steels or iron | 300-350 | The second name is bluing. A very common method, its main purpose is decorative metalworking, since the part acquires a black (raven) color. An example of application is the creation of small arms. Another advantage is that the original dimensions are preserved, because the oxide film formed is very thin, no more than one to one and a half microns. |
| Alloy steel elements | up to 700 | Application of the composition takes a long period - at least 1 hour. |
| Iron-nickel magnetic alloys | 400 – 800 | The process lasts for 0.5 - 1.5 hours. A layer appears that is considered a dielectric, so it is simply necessary when creating electrical semiconductors. |
| Silicon | 800 – 1200 | The procedure is called thermocompression. It passes under high pressure up to 107 pa. The products exposed to it are necessary in electronics. |
Pulsed laser radiation
When heating occurs not in a furnace, as with the thermal method, but with the help of a laser, the result is good, although the process is more difficult. Research is still being carried out on which materials should be properly exposed to the beam, but one option is pulses - that is, a short flow of flow to an area with a gradual displacement of the installation head.
Continuous radiation
In this case, only durable steels that are not afraid of overheating under constant exposure are processed. A beam is directed at the zone, which continuously moves throughout the entire oxidation area. Accordingly, the heating is very significant.
Problems and difficulties of work on oxidation of stainless steel
The information presented in this article will be useful for materials scientists, technologists and engineers involved in the processing and study of the properties of metals. The production of decorative coatings is increasingly being used in various branches of mechanical engineering, the automotive industry, and in the production of household items. Let's consider one of the most popular methods of creating a film - oxidation of stainless steel.
With your own hands
The methods presented above are used only in production, but if you are ready for independent experiments, then you need to create a small home laboratory.
For the experiment, take a small steel piece that will fit into a three-liter jar without any problems.
Stages of work
Do each of them consistently and thoroughly. Prepare all the necessary tools in advance.
Rough stripping
Use a steel brush or coarse sandpaper. You need to remove all rust down to the base, as well as other contaminants. It is better if you then go over it with fine-grained sandpaper to ensure a uniform surface.
Polishing
Special pastes with fine abrasives or discs on hand-held grinders are perfect.
Plaque removal
In other words, rid the element of grease, oil traces, and polishing paste residues.
Treatment
To do this, introduce a solution of sulfuric acid with a 5% content of the substance and place the workpiece there for 1 minute.
Washing
First, rinse the part in regular running water, and then boil it in a soapy water mixture. Now make a 5% solution of caustic soda in a container, place the workpiece there and heat to 150 degrees, hold for 2 hours. Then just let it cool and evaluate the result. You've got an oxidized coating - this is a wonderful effect achieved at home. To clarify the information you are interested in and purchase Russian-made metal bandsaw machines, contact the managers by phone 8 (908) 135-59-82;;. They will answer all your questions.
Architectural anodizing
Architectural anodizing produces a finish that is harder than glass, meaning it is less susceptible to damage, wear, and can be sanded down if necessary to restore its original shine. Advantages of anodized aluminum in architecture:
Aesthetics
The clear oxide layer highlights the rich metallic appearance of the aluminum rather than hiding it like paint. The oxide layer, unlike powder painting, does not flake off or peel off.
Corrosion resistance
The oxide layer is resistant to corrosion and this is one of the most important advantages of anodized aluminum.
The aluminum oxide layer is durable, hard and self-renewing because aluminum spontaneously forms a thin but effective protective oxide layer that prevents further oxidation or corrosion when mechanically damaged.
Anodized aluminum will not patina like copper and zinc, and will not rust like steel. It is an excellent material for use in marine and coastal environments.
Anodized aluminum is highly weather resistant, even in many industrial environments where other metals often corrode. The main pollutants in urban environments are carbon monoxide and carbon dioxide, which do not affect anodized aluminum surfaces.
Durability
Featuring a highly durable and abrasion-resistant oxide layer, anodized aluminum is strong enough to withstand harsh and hostile climates.
Resistance to mechanical damage
Aluminum oxide is a very hard compound, ranked second only to diamond on the Mohs scale of mineral hardness. Therefore, the anodized aluminum surface provides excellent scratch and abrasion resistance.
No peeling
Anodizing is an electrolytic process that converts the surface of a metal into an oxide layer integrated into the metal itself. It is not a coating applied to metal surfaces. Consequently, there is no risk of destruction of the anode film associated with processes such as dusting, the formation of bubbles, cracks, chips or peeling.
No fading
Shades such as silver, champagne, bronze, gold and black do not contain organic elements. These coatings do not fade throughout their entire service life.
No dust
Dusting is the formation of a fine powder on the painted surface of a film under the influence of atmospheric phenomena (grains of sand carried by the wind). It can cause significant deterioration in surface appearance with reduced gloss, surface gloss and color.
Anodized aluminum is not subject to this problem: it is resistant to negative environmental influences and is equally stable in hot (desert), sea or humid climates.
No filamentous corrosion
Filiform corrosion is an attack on the hidden area between the aluminum and the paint layer, causing corrosion to spread beneath the paint layer.
When anodizing, the anodic (oxide) layer is integral with the aluminum, and the interlayer layer is simply absent. This means that the coating will never be subject to thread-like corrosion.
Moreover, in the event of surface damage from an impact or puncture, aluminum will simply restore itself through natural oxidation.
Uniform coverage
When anodizing, the product is completely immersed in the bath, which ensures uniform coverage of the surface with an oxide film.
Applicable devices and equipment
On an industrial scale, a sulfuric acid solution is used for anodizing steel, which ensures a high process speed and the greatest penetration depth.
Modern installations are fully automatic lines with a minimum number of personnel, whose role is reduced to monitoring the work process. All equipment can be divided into three types:
- Basics. It includes a bath and a cathode. The container must be made of an inert material with high thermal insulation properties - in this case, the electrolyte will not heat up too quickly and will last much longer. The cathode material depends on the type of metal being processed. For example, to anodize aluminum, a lead sheet is used, the size of which should be twice the dimensions of the workpiece.
- Serving. This includes units that are responsible for ensuring the operation of the installation: drive mechanisms and devices for transmitting current.
- Auxiliary. We are talking about equipment on which work is carried out to prepare workpieces for anodizing. This also includes mechanisms for moving parts and storing them.
When selecting a suitable installation, the following features must be taken into account:
- The most labor-intensive operations are loading and unloading the workpiece. Pay attention to the reliability and power consumption of these nodes.
- Productivity depends on the power of the power plant. As practice shows, the optimal rectifier power is 2.5 kW. The presence of stepless voltage level adjustment will be an additional advantage, facilitating the process of anodizing steel.
Stepless regulation will occur after the formation of a protective layer of medium thickness, when in order to maintain the current level it will be necessary to gradually increase the voltage.
- Contact pads made of flexible material should be installed along the rings of the container. Copper elements will cope best with this task.
Technology
To carry out work on an industrial scale, special galvanic workshops and production facilities are created, which are considered “dirty” and harmful to human health.
Therefore, recommendations for carrying out the process at home, advertised in some sources, should be taken with extreme caution, despite the apparent simplicity of the technologies described
An anodized coating can be created in several ways, but the general principle and sequence of work remain classic. In this case, the strength and mechanical properties of the resulting material depend on the original metal itself, the characteristics of the cathode, the current strength and the composition of the electrolyte used. It must be emphasized that as a result of the procedure, no additional substances are applied to the surface, and the protective layer is formed by transforming the original material itself. The essence of galvanics is the effect of electric current on chemical reactions. The whole process is divided into three main stages.