Aluminum products are used in all spheres of human activity. The sought-after metal has a lot of valuable advantages, but anodizing aluminum allows you to make it even better. We will understand how anodized aluminum is produced, what advantages it acquires, and what types of technology exist.
Aluminum sheet with anode coating Source tildacdn.com
Principles of the anodizing process
The process of electrochemical oxidation of aluminum and its alloys in solutions of sulfuric, chromic, oxalic acids and their mixtures is called aluminum anodization. Despite its apparent simplicity, the anodizing process has many options that directly affect the characteristics and quality of the oxide film. The appearance and structure of the coating is also affected by the composition of the aluminum alloy, and adjusting the electrolyte allows you to change the properties of the coating within a wide range. The quality and presence of impurities in the electrolyte composition can also be critical.
Anodizing differs significantly from the processes of electroplating metals (electrochemical deposition), in which a protective or decorative layer of metal is applied to the surface of a metal product, as it is a process of transforming the base metal, as a result of which the appearance and characteristics of the surface change.
Cold anodizing
Technologically, the process is similar to the previous option, the only difference is that such anodization occurs at a low temperature, in the range from -10 to +10 °C. The advantage of this method is that the protective film is thick and durable. The cold environment acts so that the layer grows faster on the inside than it dissolves on the outside.
The processed product is highly resistant to corrosion. The technique has a drawback - it is almost impossible to qualitatively paint anodized metal with organic compounds.
Painting improves the quality and aesthetics of the surface Source gidpokraske.ru
Application of Anodizing
The use of anodization is a topic for a separate article; in any industry where products made of aluminum or its alloys are used to one degree or another and any properties of the metal need to be changed, anodization is the optimal and often the only solution.
Here is a list of the main areas of application of anodizing:
- Thin oxide films are used as a basis for applying organic and inorganic coatings (paint or varnish).
- Color anodizing. The use of various coloring electrolytes makes it possible to obtain a wide range of shades and colors of the surface of an aluminum product. Nickel, cobalt or tin salts are used as additives. The resulting shades range from light bronze to black.
- Increased wear resistance. Oxide coatings on aluminum are much harder than the base metal. Hard anodizing is widely used for parts subject to abrasion under light loads, as well as to increase the corrosion resistance of products.
- Electrical insulation. Compared to organic insulating materials, oxide film not only has high insulating properties, but also has significantly greater heat resistance.
- Obtaining a compacted surface with high anti-friction properties. (lubricating coating).
Briefly about the main thing
Anodizing aluminum improves and expands the performance characteristics of the metal. The essence of the process is the growth of an oxide film, the properties of which depend on the method of its preparation. In industrial conditions, hard anodizing is used, the oxide coating is durable and wear-resistant.
Warm anodizing allows you to obtain a not very strong porous structure, which, however, has good adhesion and can be painted with high quality. The result of the cold method is a thick layer of oxide with high anti-corrosion properties.
Selecting anodizing electrolyte
As mentioned above, the properties of the oxide film obtained by anodization are influenced by many factors - the type of aluminum alloy, the method of pre-treatment of the surface of the part, the anodizing mode and the type of finishing operations. The composition of the electrolyte is also decisive. Acid electrolytes are mainly used (alkaline electrolytes can be used in some cases for special types of anodizing). The main acid is sulfuric; the vast majority of anodizing electrolytes are prepared on its basis. Other acids are used to obtain special types of coatings.
Possibility of using anodized aluminum
Anodized parts are used in a wide variety of applications. This method is used to process interior items, dishes, handrails and other products that are used every day. This process is also used for suspended aluminum facades - they acquire increased resistance to external atmospheric influences.
Anodizing is used to protect parts of various equipment from corrosion. These are components for cars, airplanes, ships, and all kinds of aircraft. The treatment increases strength and provides increased resistance to stress.
Anodizing in sulfuric acid electrolyte
Anodizing in sulfuric acid makes it possible to obtain translucent, colorless coatings with a thickness of about 35 microns. If the anodizing process is preceded by a process of glossing the surface of the parts, the coatings obtain high decorative qualities (shiny anodizing). In sulfuric acid, plastic anodic films are also obtained, which are not destroyed when molding products.
Sulfuric acid concentration and electrolyte temperature
The concentration of sulfuric acid for anodizing in industrial conditions is taken in the range of 8-35% (by weight). In a concentrated solution, the anodic film becomes soft and porous, and the elasticity of the film is high. The classic concentration is 15% (by weight). The temperature during the anodizing process is set in the range from 180C to 250C. In most cases, a temperature of 200C is accepted. Using sulfuric acid, solid anodic films are also obtained; in this case, the anodizing process is carried out at low temperatures (from -5 to +5 0C).
Temperature control during the anodizing process is mandatory; the current density and the rate of film dissolution depend on the temperature, which in turn has a direct impact on the quality and characteristics of the coating. In order to avoid local overheating of the electrolyte solution, special mixing devices are used.
Voltage and current density
When anodizing in sulfuric acid, a standard rectifier with an output voltage of up to 24 volts is used. In standard mode, the current is 16 volts with a current density of 1.5 A/dm2. To obtain corrosion-resistant films of large thickness, the voltage and current are raised to 18 volts, and when processing aluminum-silicon alloys to 22 volts. In some cases, for example, when anodizing rolled material or wire, alternating current is used. The use of a reduced current density makes it possible to obtain thin, transparent oxide films that are superior in transparency to films of similar thickness obtained at standard current densities.
Process duration
The duration of the anodizing process depends on the required film thicknesses, as well as the current density used. For pure aluminum this ratio can be proposed as:
Film thickness, microns. = (Current density, a/dm2 X Time, min.)/3
The ratio is approximate, since the duration of the process may depend on the type of alloy and processing mode.
The working process
The technological process of anodizing differs from the processes of applying galvanic coatings primarily in that the dissipative ability of anodizing electrolytes is significantly higher than that of electrolytes used in the processes of chrome plating, copper plating, galvanizing or nickel plating of metal. Effective dispersive ability with active mixing makes it possible to obtain films of uniform thickness over the entire surface of the products, including the internal surfaces of holes and grooves.
Otherwise, the technological process of anodizing is similar to the processes of electrochemical coating - products are immersed in a preheated electrolyte on hangers or clamps, the parts do not come into contact with each other, the distance to the cathode must be at least 15 cm (for larger products, the values are higher). Then the solution is stirred and current is applied. Under normal conditions, the cathode area should be equal to the anode area, and the cathode cross-section should be sufficient to ensure the required current density.
At the end of the process, stop the current supply and immediately remove the products from the galvanic bath. The products are washed in running water and dried.
Preparatory process
To obtain a smooth surface, the workpiece must be polished at the preparation stage. Using a felt or other polishing wheel, scratches are removed and large pores are tightened. The absence of micro-irregularities reduces the likelihood of burnouts. The anodic film is not able to hide external defects.
Before anodizing aluminum, it is necessary to determine the dimensions of the parts to be processed. The resulting layer is 50 microns thick, so it will be impossible to screw a nut onto the treated thread. If the parts are connected using a fit, then do not forget that after anodizing the parts cannot be ground.
Carrying out anodizing at home
To carry out the process, containers are needed. Containers for anodizing must correspond to the dimensions of the parts, and be slightly larger. Therefore, they usually use several baths. The material of the containers is aluminum. But if the products are small, then plastic containers are suitable. Only aluminum sheets need to be laid on the bottom and along the walls. This is necessary to create a current of uniform density throughout the entire volume.
The electrolyte needs to be insulated from external heat. When heating it will have to be changed. To prevent heating, the outside of the container is covered with a layer of thermal insulation. It can be covered with polystyrene foam up to 50 mm thick or, placed in a box, the free space can be filled with polyurethane foam.
A sulfuric acid solution is prepared by diluting the electrolyte for car batteries with distilled water in proportions of one to one. By purchasing a canister with a capacity of 5 liters, you can get 10 liters of solution.
Mixing, when water is added to the acid, is accompanied by abundant heat, and it literally boils and splashes. Therefore, for safety reasons, sulfuric acid is poured into a container of water.
Before anodizing aluminum begins, it is subjected to chemical preparation. Chemical preparation is a degreasing process. In industrial conditions, treatment is carried out with caustic soda or potassium. But at home it is better to use laundry soap. A toothbrush and soap solution can easily remove dirt from the surface. After that, the workpieces are first washed with warm water, and then with cold water.
An alternative to laundry soap is washing powder. After dissolving it in a closed plastic container and placing the parts to be processed there, shake vigorously. Then the parts are washed and dried with a stream of hot air. The active oxygen contained in the washing powder protects fat-free products, even if handled with bare hands.
Anodizing in chromic acid
Chromic acid is used if it is necessary to anodize critical aluminum parts and assemblies with thin walls or with high processing precision. The dissolution of aluminum in chromic acid is lower than in sulfuric acid, the reduction in the fatigue strength of the metal is lower - the film turns out to be thin, opaque gray in color. The maximum thickness of the oxide film reaches 10 microns, the standard thickness is from 2.5 to 5 microns.
The concentration of chromic anhydride CrO3 is taken in the range from 2 to 15% (by weight). In most cases, the regime temperature is set within the range of 25-400C; active stirring of the electrolyte solution is not required. When anodizing in a 10% solution of chromic acid, the process temperature is raised to 540C at a voltage of 30 volts to ensure a current density of 1.2 A/dm2. For alloys containing copper or zinc, the voltage is set within 15-20 volts at the same current density. When anodizing in an electrolyte of low concentration 3-5% (by mass), a special voltage supply mode is used and the process proceeds in cycles. This mode is used to detect defects in the surface of a product or when forming a sublayer for painting.
Warm anodizing
The warm anodizing method is used to obtain a base for painting. The coating is porous, but due to this it has high adhesion. Epoxy paint applied on top will reliably protect the aluminum from external influences.
The disadvantage is the low mechanical strength and corrosion resistance of the coating. It is destroyed upon contact with sea water and active metals. This method can be done at home.
The process takes place at room temperature or higher (no more than 50°C). After degreasing, the workpieces are mounted on a suspension that holds them in an electrolyte solution.
Anodizing continues until a milky coating appears on the surface. After removing the stress, the workpieces are washed in cold water. The parts are then painted. They are dyed by placing them in a container of hot dye. After that, the obtained result is consolidated for 1 hour.
Methods for color anodizing of aluminum
Anodization in oxalic acid
In a solution of oxalic acid, yellow films with high wear resistance are obtained. This method is one of the first open methods for obtaining a colored coating. The wear resistance of the coating during abrasion is two times higher than when anodizing in sulfuric acid. In the process of anodization in oxalic acid, along with direct current with a voltage of 30-60 volts, alternating current modes are used. To obtain a uniform yellow or bronze tint, the solution is vigorously mixed. Otherwise, this process is no different from anodization in sulfuric acid. Various metals can be used as cathodes - iron, lead, stainless steel.
Cold technology
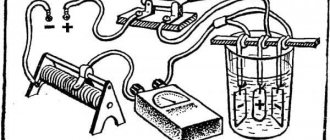
To anodize aluminum you need:
- power supply 12 V (battery, stabilizer);
- aluminum wires;
- rheostat;
- ammeter;
- containers for solutions.
Cold technology is different in that the growth of the anodized coating on the metal side proceeds at a faster rate than its dissolution on the outside.
First, the preparatory work described above is carried out. Then the parts need to be secured. It should not be forgotten that a film does not form under the fastening element. And suspended workpieces, when lowered into the container, should not touch the walls and bottom.
The anode is connected to the parts from the power source, and the cathode is connected to the capacitance. The current density is selected within the range of 1.6-4 A/dm2. Recommended values 2-2.2 A/dm2. At small values, the process will proceed more slowly, and at large values, a breakdown of the circuit may occur and the coating will begin to collapse.
It is not recommended that the electrolyte temperature rise above 5°C. When anodizing, the electrolyte does not heat up evenly. It is warmer in the center than in the corners of the container, so constant stirring is necessary.
The duration of anodizing using the cold method is about half an hour for small elements. For large parts, the duration may be 60-90 minutes. The end of the process is indicated by a changed color on the surface of the aluminum product. After disconnecting the wires, the part is washed.
Other anodizing solutions
In some cases, electrolytes are used in which the aluminum oxide film does not dissolve - so-called barrier-type electrolytes . Using anodizing solutions containing boric acid, ammonium tartrate, and ammonium borate, coatings are obtained on parts used in electrical appliances (electrolytic capacitors). For example, when treated in a solution with ammonium borate, films are obtained with a breakdown voltage of 550 volts. Also, these types of electrolytes are used in anodizing aluminum deposited in a vacuum.
Aluminum parts, the processing of which involves the application of galvanic coating after anodizing, are processed in a solution containing 25-30% phosphoric acid. The resulting films have a thickness of up to 6 microns, which is due to the high solubility of aluminum in phosphoric acid. The process is carried out at workshop temperature, current density 10-20 A/mm2 and voltage 30-60 volts for 10-15 minutes.
Solid films of golden, brown or black colors are obtained by using a solution containing 40-100 g/l sulfosalicylic acid and 30-60 g/l sulfuric acid at a temperature of 300C, a current density of 2.5-3.5 a/dm2 and a voltage of up to 80 volts.
Electrolyte preparation
Acid solutions are considered unsafe reagents, so to anodize aluminum at home, they resort to a different type of solution. To prepare it, use salt and soda, which you always have on hand.
To prepare the electrolyte, take two plastic containers. They are filled with salt and soda compositions, observing the proportion: per serving of salt or soda 9 servings of distilled water.
Anodizing at home
After dissolving the components, the solution is kept to allow undissolved particles to settle to the bottom. When pouring into a container for anodizing, it must be strained.
Removing anodic coatings
Poor-quality anodic coating can only be removed from the entire surface of the product; partial restoration of the film is impossible in most cases. The coating is usually removed in solutions containing caustic alkalis. The process takes place under strict control of the main modes, since such solutions have a high degree of impact on the base metal. The classic solution that has the least impact on the aluminum surface is one containing 35 ml/l phosphoric acid and 20 g/ml chromic acid. Treatment takes place within 1-10 minutes, depending on the thickness of the film at a temperature of 95-1000C. to remove hard anodic coatings, use the specified solution with a concentration twice as high, while the surface of aluminum alloys containing copper can be painted gray or black.
Re-processing of products after removing the anodic film is possible after assessing the condition of the surface of the product; if the surface cleanliness is sufficient for coating and polishing is not required, you can begin the process immediately.
It should be noted that when processing parts for which it is necessary to strictly adhere to the original dimensions, repeated anodization will be required with the application of a film of greater thickness than it was originally. This is because when the coating is removed and reapplied, losses can range from half to two-thirds of the original film thickness.
You may be interested in the following articles:
|
Where is anodized metal used?
The technology has improved the original properties of the metal. Anodized aluminum is used in a wide variety of technical fields and allows you to achieve different goals, for example:
- Corrosion protection of architectural structures. Aluminum structures gained popularity in the 1960s, and anodizing soon replaced liquid painting. The standard layer thickness in different countries is 15-25 microns, depending on environmental conditions.
- Use of reflective properties. The shiny surface has found application in various fields of technology, from thermal reflectors (in heating reflectors), to spotlight reflectors and lighting elements. A layer 1-2 microns thick easily tolerates high humidity and temperature.
Reflective properties are in demand in the production of floodlights Source cepolina.com
See also: Catalog of companies that specialize in finishing materials and related work
- Resist wear, reduce friction. The smooth coating significantly reduces wear and increases the hardness of friction parts. Therefore, a layer up to 60 microns thick is used to coat parts of mechanisms and engines.
- Creation of a dielectric film. An electrical insulator in the form of an anodized aluminum layer is used in electrolytic capacitors and in some types of transformers.
- Particularly hard microfilm has found application in aircraft and shipbuilding, and in construction (building profiles).
- Oxide films are needed in the production of heating and cooling devices.
- Improving product quality. Anodizing an aluminum window profile improves its appearance, masks minor defects, that is, improves the quality of the product.
- Variety of designs. The process allows you to obtain anodized coatings of different colors (including imitation bronze, silver, gold). This increases the attractiveness of products (for example, hinges, handles, balustrades) and allows them to fit more accurately into the interior.
Aluminum sheets in matte silver, bronze, gold Source aeroexpo.online
- Maintaining cleanliness. A stepladder made of unprotected aluminum will get your hands dirty. Therefore, manufacturers usually strive to protect products such as handles, railings, and knitting needles with an anodic coating.
Details
Preparation process
In order to obtain a smooth surface at the preparation stage, the workpiece should be polished. Using a felt or other polishing wheel will remove scratches, and will also tighten large pores. The absence of microscopic irregularities reduces the likelihood of burnouts. The anodic film cannot hide external flaws. Before anodizing begins, you should decide on the size of the parts to be processed. The resulting layer has a thickness of 50 microns, and therefore it is impossible to screw a nut onto the treated thread. If all parts are connected by fit, then we should not forget that after anodizing the parts cannot be ground.
To carry out the process, containers are required. To carry out anodizing, they must necessarily correspond to the dimensions of the elements, and be slightly larger. In this regard, several baths are usually used. The material used to make the container is aluminum. But if the products are small, then plastic containers are also suitable. Only aluminum sheets need to be laid on the bottom and along the walls. This is required in order to create a current of a uniform plane throughout the total volume as a whole. The electrolyte needs to be insulated from external heat. When warming up, it needs to be changed. To prevent heating of the container, the outside should be covered with a layer of thermal insulation. It can be covered with polystyrene foam up to 5 cm thick, or placed in a box, filling the free space with mounting foam.
Please note that for anodizing at home, a sulfuric acid solution is obtained by diluting the electrolyte for car batteries and distilling it with water in a ratio of 1 to 1. By purchasing a canister with a volume of 5 liters, you can get 10 liters of solution.
Mixing, when a little water is added to the acid, is accompanied by a strong release of heat, and it literally begins to boil and splash. For this reason, for safety reasons, sulfuric acid is poured into a canister of water. Before the anodizing process begins, it is subjected to chemical preparations. We are talking about the degreasing process. In industrial conditions, they are treated with potassium or caustic soda. But at home it is better to use regular laundry soap. Using a soap solution and a toothbrush, dirt should be thoroughly removed from the surface. After this, to begin with, the workpieces should be rinsed with warm water, and then cold. By the way, washing powder can be an alternative to laundry soap. It should be dissolved in a closed plastic container and the parts to be processed should be placed there, shaken vigorously. Next, the parts are washed and dried with a hot air stream. Active oxygen, which is contained in washing powder, also protects low-fat products, even if you handle them without protective gloves.
Electrolyte preparatory stage
Acid solutions can be considered unsafe reagents, and therefore, to anodize aluminum metals at home, they resort to the rest of the solution. To prepare it, use soda and salt, which you always have on hand. To make an electrolyte, you need to take a couple of plastic containers, and add soda and saline solutions into them, observing the proportion - for 1 measure of salt or soda, add 9 portions of distilled water. After the components have dissolved, the solution should be kept to allow the dissolved particles to settle to the bottom. When pouring into a container for anodizing, it should be strained.
Aluminum anodizing methods
Several methods have been created to process aluminum alloys, but it is the chemical method in an electrolyte environment that has found widespread use. In order to make such a solution, the following oxygens are used:
- Sorrel.
- Sulfur.
- Chrome.
- Sulfosalicylic acid.
In order to impart additional properties to the solution, organic acids or even salts. At home, sulfuric acids are mainly used, but when processing parts with complex configurations, it is preferable to use chromic acid. The process is carried out at temperatures from 0 to +50 degrees. At low temperatures, a hard coating forms on the aluminum surface. As the temperature level increases, the process begins to proceed much faster, but the coating will have a high degree of softness and porosity.
In addition to chemical methods, in certain cases the following methods of anodizing aluminum are used:
- Micro-arc.
- Colored - by dipping into an electrolyte, by adsorption. By immersion in a dyeing solution and electroplating.
- Integral.
- Interferential.
Now let's look at a couple more methods in more detail.
Thermal anodizing method
Anodizing steel at home (and warm) is used to obtain a base for paint. The coating is porous, but due to this it will have a high degree of adhesion. Epoxy paint applied on top will reliably protect steel and aluminum from external influences. The disadvantage will be the low mechanical strength and corrosion resistance of the coating. It is destroyed upon contact with sea water and active metals. This method can be done at home. The process will take place at room temperature or even higher (but not more than +50 degrees). After degreasing, the workpieces are mounted on hangers that will hold them in the electrolyte solution.
Anodizing continues until a milky coating is formed on the surface. After removing the stress, the workpieces should be washed in cool water. Next, the elements must be painted. Dye them by placing them in a container with hot dye. After this, the result should be consolidated for 60 minutes.
Cold way
To anodize a metal such as aluminum, you need:
Power supply 12 V (stabilizer or battery).- Rheostat.
- Aluminum wires.
- Containers for solution.
- Ammeter.
By the way, cold technology will differ in that the growth of an anodized coating on the metal side proceeds at a greater speed than its dissolution on the outside. First you need to carry out the preparatory work described earlier. Next you need to secure the parts. Don’t forget also that a film does not form under the fasteners. And suspended workpieces, when lowered into the container, should not touch the bottom and walls. The anode should be connected to the elements from the power source, it turns out, to the capacitance under the cathode. The current density should be selected within the range of 1.6 d 4 A/dm2. Recommended value is from 2 to 2.2 A/dm2. With a low value, the process will proceed much more slowly, and with a large value, a breakdown may occur in the circuit and the coating begins to collapse.
It is not recommended that the electrolyte temperature rise above +5 degrees. When anodizing, the electrolyte will heat up unevenly. It will be warmer in the center than in the corners of the container, so constant mixing is required.
The duration of the anodizing process using the cold method is approximately ½ hour for a small element. For large parts, the duration can be from 1 to 1.5 hours. The end of the process is indicated by a changed shade on the surface of the aluminum product. After disconnecting the wires, the part should be washed.