A piece of pure aluminum
Aluminum
- a very rare mineral of the copper-cupalite family, a subclass of metals and intermetallic compounds of the class of native elements. Mainly in the form of microscopic deposits of a continuous fine-grained structure. It can form lamellar or scaly crystals up to 1 mm, whiskers up to 0.5 mm long are noted. with a thread thickness of several microns. A lightweight paramagnetic metal of silver-white color, easy to form, cast, and machine.
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Ice
– structure and physical properties
Copper
– structure and physical properties
STRUCTURE
Cubic face-centered structure. 4 orange atoms
The crystal lattice of aluminum is a face-centered cube, which is stable at temperatures from 4°K to the melting point. There are no allotropic transformations in aluminum, i.e. its structure is permanent. The unit cell consists of four atoms with a size of 4.049596×10-10 m; at 25 °C, the atomic diameter (the shortest distance between atoms in the lattice) is 2.86 × 10-10 m, and the atomic volume is 9.999 × 10-6 m3/g-atom. Impurities in aluminum have little effect on the lattice parameter. Aluminum has great chemical activity; the energy of formation of its compounds with oxygen, sulfur and carbon is very high. In the voltage series, it is among the most electronegative elements, and its normal electrode potential is -1.67 V. Under normal conditions, interacting with atmospheric oxygen, aluminum is covered with a thin (2-10-5 cm) but durable film of aluminum oxide A1203, which protects against further oxidation, which determines its high corrosion resistance. However, if Hg, Na, Mg, Ca, Si, Cu and some other elements are present in aluminum or the environment, the strength of the oxide film and its protective properties are sharply reduced.
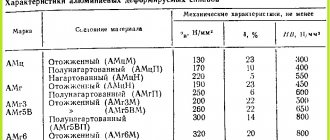
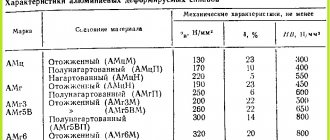
Technical parameters of aluminum-based alloys
The presence of a ligature in the composition has virtually no effect on the elasticity of the material, but increases fluidity, which makes it possible to use it for the production of structures with different load levels.
The strength or resistance of a material to fracture or deformation under mechanical loads depends on the type of treatment and its composition. For metal alloys it is 38–42 kg/mm², cast aluminum 10–12 kg/mm², deformable – 18–25 kg/mm².
Pure material has high plasticity, and the presence of alloy components changes the properties of the composition, which allows the material to be used in different areas of production.
Most alloys with a higher degree of alloying have low electrical conductivity. The thermal conductivity of many compositions is half that of pure aluminum, but this figure is higher than that of steel.
The most well-known aluminum alloys are the following compositions:
- duralumin, including alloy additives of copper and magnesium;
- silumin - a compound with silicon.
Aluminum, heat-intensive and ductile, forms various alloys
The resistance of the material to environmental influences is increased by adding gallium, tin, and indium. Alloys with manganese and magnesium have the best corrosion properties, while compositions with high strength have the worst.
Depending on the nominal lithium content, the density of the material changes. With 1.3% lithium, the density is 2.59 g/cm³, 2.2% – 2.58 g/cm³, 2.0% – 2.55 g/cm³.
Resistance to external conditions depends on the processing mode of the material. Many heat-strengthened compounds are susceptible to stress corrosion.
Among aluminum-based compositions, avial is well welded - aviation aluminum, which contains magnesium, silicon and impurities of manganese, copper and chromium. Spot welding is used for most alloys.
As the degree of alloying increases, the strength of materials increases and ductility decreases. As temperature increases, the strength of materials changes to varying degrees, which determines their use depending on the temperature range.
The type of hardening of the compositions improves the mechanical properties of the material: pressed products have higher strength than hot-rolled ones.
PROPERTIES
Native aluminum. Field of view 5 x 4 mm. Azerbaijan, Gobustan region, Caspian Sea, Here-Zira or Bulla Island
Aluminum is a soft, lightweight, silver-white metal with high thermal and electrical conductivity, and is paramagnetic. Melting point 660°C. The advantages of aluminum and its alloys include its low density (2.7 g/cm3), relatively high strength characteristics, good thermal and electrical conductivity, manufacturability, and high corrosion resistance. The combination of these properties allows us to classify aluminum as one of the most important technical materials. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it becomes covered with a protective oxide film - aluminum oxide) and reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide. Aluminum dissolves even in dilute hydrochloric and sulfuric acids, especially when heated. But aluminum does not dissolve in highly diluted and concentrated cold nitric acid. When aqueous solutions of alkalis act on aluminum, the oxide layer dissolves, and aluminates are formed - salts containing aluminum as part of the anion.
Metal density indicator
The density parameter of any substance is calculated as the ratio of mass to volume and measured in g/cm³. Using this indicator for arithmetic calculations allows you to determine the weight of workpieces or products.
Often, to estimate the amount of material per unit volume, the specific gravity indicator is used, which, unlike density, has only a quantitative characteristic.
Aluminum, whose density is 2712 kg/m3, is the most popular material for various industrial sectors. Due to its special physical and chemical characteristics, the metal is used as a alloy component of an alloy with gold.
The melting point is 660 °C. Metal boils at a temperature of 2519 °C. The density of liquid metal is 2560–2640 kg/m3, in the solid state the figure is 2712 kg/m3. Molten chemically pure metal at a temperature of 660 °C has a density of 2.368 g/cm³, and at 1173 °C - 2.304 g/cm³.
Aluminum has high thermal conductivity, which is taken into account along with the physical parameters of the composition. The density of aluminum alloys differs slightly from the density for pure metal.
RESERVES AND PRODUCTION
Aluminum pieces
In terms of prevalence in the Earth's crust, it ranks 1st among metals and 3rd among elements, second only to oxygen and silicon. The mass concentration of aluminum in the earth's crust, according to various researchers, is estimated from 7.45 to 8.14%. The modern production method, the Hall-Héroult process, was developed independently by the American Charles Hall and the Frenchman Paul Héroult in 1886. It consists of dissolving aluminum oxide Al2O3 in molten cryolite Na3AlF6, followed by electrolysis using consumable coke or graphite anode electrodes. This production method requires very large amounts of electricity, and therefore received industrial application only in the 20th century.
Brief description of the chemical properties and density of aluminum
When finely crushed aluminum is heated, it burns vigorously in air. Its interaction with sulfur proceeds similarly. The combination with chlorine and bromine occurs at ordinary temperatures, and with iodine - upon heating. At very high temperatures, aluminum also combines directly with nitrogen and carbon. On the contrary, it does not interact with hydrogen.
2Al + 3F2 = 2AlF3 (to = 600 o C);
2Al + 2S = Al2S3 (to = 150 – 200 o C);
2Al + N2 = 2AlN (to = 800 – 1200 o C);
4Al + P4 = 4AlPt o = 500 – 800 o C, in H2 atmosphere);
4Al + 3C = Al4C3 (to = 1500 – 1700 o C).
Aluminum is almost completely resistant to water. Highly diluted and very concentrated solutions of nitric and sulfuric acids have almost no effect on aluminum, whereas at average concentrations of these acids it gradually dissolves.
Read also: How to tighten a belt with two rings
Aluminum is resistant to acetic and phosphoric acids. Pure metal is also quite resistant to hydrochloric acid, but ordinary technical metal dissolves in it. Aluminum is easily soluble in strong alkalis:
ORIGIN
Aluminum aggregated with a bayerite crust on the surface. Uzbekistan, Navoi region, Uchkuduk
Due to its high chemical activity, it is not found in pure form, but only as part of various compounds. For example, there are many known ores, minerals, and rocks that contain aluminum. However, it is extracted only from bauxite, the content of which in nature is not very high. The most common substances containing the metal in question are: feldspars; bauxite; granites; silica; aluminosilicates; basalts and others. In small quantities, aluminum is necessarily found in the cells of living organisms. Some species of club mosses and marine inhabitants are capable of accumulating this element inside their bodies throughout their lives.
Density of aluminum and its other physical properties
At room temperature, aluminum does not change in air, but only because its surface is covered with a thin film of oxide, which has a very strong protective effect.
Read also: Jet for a gas boiler
Rice. 1. Aluminum. Appearance.
Aluminum is characterized by high ductility and high electrical conductivity, approximately 0.6 of the electrical conductivity of copper. This is due to its use in the production of electrical wires (which, with a cross-section that ensures equal electrical conductivity, are half the weight of copper). The most important aluminum constants are presented in the table below:
Table 1. Physical properties and density of aluminum.
Density, kg/m 3
Melting point, o C
Boiling point, o C
Atom ionization energy, eV
Atomic radius, nm
Standard enthalpy of dissociation of molecules at 25 o C, kJ/mol
APPLICATION
Aluminum decoration
Widely used as a construction material. The main advantages of aluminum in this quality are lightness, malleability for stamping, and corrosion resistance. The electrical conductivity of aluminum is only 1.7 times less than that of copper, while aluminum is approximately 4 times cheaper per kilogram, but due to its 3.3 times lower density, to obtain equal resistance it needs approximately 2 times less weight . Therefore, it is widely used in electrical engineering for the manufacture of wires, their shielding, and even in microelectronics when depositing conductors on the surface of microcircuit crystals. When aluminum was very expensive, a variety of jewelry was made from it. Thus, Napoleon III ordered aluminum buttons, and in 1889 Mendeleev was presented with scales with bowls made of gold and aluminum. The fashion for jewelry made of aluminum immediately passed when new technologies for its production appeared, reducing the cost many times over. Nowadays, aluminum is sometimes used in the production of costume jewelry.
Aluminum – Al
| Molecular weight | 26.98 g/mol |
| origin of name | from Latin alumen |
| IMA status | approved in 1978 |
Specific heat capacity of aluminum
The specific heat capacity of aluminum depends significantly on temperature and at room temperature is about 904 J/(kg deg) , which is significantly higher than the specific (mass) heat capacity of other common metals, such as copper and iron.
Below is a comparative table of the specific heat capacities of these metals. The heat capacity values in the table are in the temperature range from -223 to 927°C.
According to the table, it can be seen that the value of the specific heat capacity of aluminum is significantly higher than the value of this property for copper and iron , therefore this property of aluminum, such as the ability to accumulate heat well, is widely used in industry and heating engineering, making this metal irreplaceable.