Varieties
Silver-containing solders are divided into groups based on the proportion of silver they contain .
Compositions containing up to 40% base metal are used when joining parts made of steel and non-ferrous alloys. The strength characteristics of this material make it possible to solder workpieces no thicker than 3 mm.
Solders containing 40-60% Ag are suitable for soldering copper, nickel and steel in structures subject to heavy constant and variable loads.
The high content of the base metal allows the use of silver solder for critical high-load connections, contacts in electrical engineering and electronics, band saw blades and the like.
Physico-chemical properties and composition
The chemical composition of the soldering material is determined by GOST and affects its physical and chemical parameters. To change the properties, elements such as copper, zinc, tin, antimony and others are included in the composition.
Standard for dobvako content in various brands of solders.
To reduce the cost of the composition when soldering less critical compounds, silver is diluted with tin, lead, and zinc, which are cheap relative to silver.
Preparation of materials
Before soldering silver jewelry at home, you should prepare, in addition to the soldering tool, the appropriate solders and flux.
In this case, the best flux for soldering is colophonium - a special resin that forms a film over the soldering area and does not allow reaction with surrounding oxygen.
If you can’t find colophonium in stores, you can make flux for jewelry silver yourself. This flux will contain two elements:
- borax (sodium tetraborate decahydrate);
- potash (potassium carbonate).
They need to be combined in a one to one ratio. Borax for soldering should be purchased in powder form, and not in the form of an aqueous solution (otherwise it must first be dried). The process of preparing this flux is quite simple: borax crystals are ground in a mortar and mixed with potash.
The silver jewelry itself also needs preliminary preparation - it must be cleaned and treated with alcohol (degreased).
Features of choice
The correct selection of soldering material is the key to high strength and durability of the soldered seam. When choosing a brand, you should also take into account the technical and operational requirements:
- static and dynamic loads on the connection;
- temperature regime;
- the chemical activity of the environment in which the seam will work;
- planned cost of the seam.
Thus, to create high-temperature joints, copper is added to the composition, since tin and zinc have a low melting point and will weaken the joint.
The high price of the joined jewelry blanks makes it possible to maintain the economic efficiency of production when using solders with a high silver content, down to pure metal.
Application area
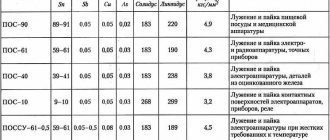
POS 40 is used for low-temperature soldering with direct use of solder. It is more economical compared to high-temperature or composite. Provides a fairly reliable and durable connection.
This additive is used to solve the following problems:
- thermal connection of metal parts, mainly brass, tin;
- connections of elements of electrical and radio-electronic equipment that do not have high thermal indicators;
- elimination of cracking and breakdowns in copper and galvanized containers that will not be subject to heating.
Solder welding POS 40
It is widely used at enterprises in the electrical and radio-electronic industries for securing parts on printed circuit boards and hardware housings. It allows for a reliable connection and excellent electrical conductivity. It is used to solder and tinning the leads of parts (resistors, capacitors, legs of microcircuits and transistors), and connecting wires. Soldering of copper cores of multicore wires and cables is carried out. The copper lugs are tinned and soldered to the cable sheath (steel or lead). Despite the difference in metals, the connection is quite strong.
It allows you to seal seams in various vessels and eliminate leaks in pipelines through which non-reactive liquids pass. Due to its low melting point, it is widely used for tinning areas of metal that need to be prepared for subsequent soldering. The presence of only tin and lead in the additive makes it possible to obtain a reliable connection after cooling. For soldering products made of brass, iron or copper, rods with a diameter of 8 millimeters are used.
Technologically, the process of tinning and subsequent soldering does not cause difficulties and does not differ from technologies for working with other solders. To obtain a high-quality connection, it is advisable to carry out preparatory work. They consist of degreasing the soldering surface and pre-tinning. The soldering iron or soldering station is selected in such a way that the melting temperature of the solder can be ensured. That is, the melting temperature of the solder must be lower than the melting temperature of the parts being soldered, otherwise not soldering will occur, but complete diffusion. In this case, you will not be able to obtain a reliable connection. For POS 40 solder, it is also necessary to take into account the solidus temperature (melting point of the lowest melting component of the additive) and the so-called liquidus temperature (the lowest temperature at which the solder becomes liquid - for POS 40 this is 238 ° C).
This temperature is sufficient to completely melt the additive, warm up the metal part well, and ensure a reliable connection due to its inherent fluidity and excellent wettability.
In addition to heating devices, to ensure high-quality soldering, it is necessary to select a flux for POS 40. When choosing a flux, the following compatibility parameters should be taken into account: type of flux, soldering temperature range, aggressiveness and state of aggregation. In practice, two types of flux are used with POS 40: active or passive. The first type allows you to remove any oxides from the metal surface. This occurs due to their dissolution. However, the surface layer of the metal itself also dissolves. Such fluxes are hydrochloric acid, zinc chloride and ammonium chloride. Passive fluxes do not have this disadvantage. With their help they only protect the place of future soldering from possible corrosion. Prominent representatives of this class are the well-known wax and rosin.
Advantages
Silver melts at 962°C . Pure silver solder allows you to create connections with high ductility. Their main advantages are:
- excellent fluidity in the molten state;
- strength;
- corrosion resistance;
- the ability to combine different metals and alloys;
- high electrical and thermal conductivity.
The melting point decreases as the proportion of silver decreases. At the same time, time and energy costs per unit of melted volume are reduced.
Silver percentage
The proportion of base metal in silver solder determines the thermal and electrical conductivity of the material.
Low
Such tin-silver solders, containing from 1.5% Ag, are popular in the electrical industry, the production of pipelines and vessels, they allow you to quickly and efficiently connect workpieces, contacts and other parts. Low resistance, high fluidity and affordable price allow the composition to be widely used.
Average
A silver share of 40-60% makes it possible to solder highly loaded connections . However, materials containing tin and silver are not recommended for use in high temperature environments. They are suitable for operation in conditions of vibration loads and chemically active environments.
High
These compounds are used for the most complex and critical compounds . Thus, the composition PSr65, containing 2% Cu and 14% Zn, is used for soldering saw blades. It is able to withstand breaking and bending forces.
PSr70 is used in the production of powerful generators, where resistance plays a decisive role.
Silver solders
Silver-based solders are the optimal solution for creating a strong, reliable weld with good electrical conductivity. In its pure form, noble metal cannot be used for soldering. It is too plastic and has a very high melting point. Therefore, other metals are added to solders, most often copper or zinc. Thanks to the additives, the melting point is lowered, and, consequently, the energy and time consumption for soldering is reduced.
Among the advantages of silver solders, we should highlight the excellent strength qualities of the resulting seams, resistance to oxidation, mechanical and vibration influences.
The number of brands of silver solders is so large that you can choose the composition for almost any task for soldering various metals.
Silver content in solder
The amount of silver in solder is regulated by GOST requirements. The product labeling contains a digital designation indicating the percentage of noble metal in the alloy. Solders with a high silver content (50-70%) are used to create seams with high electrical conductivity; alloys with less silver are recommended for joining parts that are not subject to significant heating during operation. Alloys with low Ag content are most in demand in mechanical engineering for creating high-hardness welds. Radio amateurs mainly use slavas with a reduced silver content (only about 2%).
Budget brands of silver solders
PSR-10 solder contains only 10% silver. This solder is used to create hard seams that can withstand temperatures up to 800 degrees. The materials to be soldered can be steel and alloys of non-ferrous metals, including brass with a high copper content.
Solders with a silver content of 12% are used for soldering brass (with a copper content of up to 58%) and copper.
Products with a silver content of 25% allow you to get a clean seam, however, with not the highest strength properties.
Solders with medium amount of silver
Silver solder, containing 40% silver, allows you to obtain a strong and ductile seam. Most often, this composition is used to connect moving parts, since the seam can be subject to deformation after hardening without losing its integrity.
PSR-45 solder is recommended for soldering joints of significant thickness (up to 3 mm). The seams are durable, resistant to shock and vibration loads, do not crack or oxidize
Solders with a high percentage of silver
Solder containing 65% noble metal is used to join saw blades. An alloy with a silver content of 70% is often used for soldering components in electronics. Due to the high electrical conductivity of the metal, such solder does not interfere with the conductivity of wires during soldering.
In jewelry, solders with a silver content of 70-80% have been used.
Choosing a flux for soldering
To ensure that the seam is as clean and durable as possible, the surface is treated with fluxes before soldering. Flux purpose:
- surface cleaning;
- reduction of composition oxidation;
- reduction of metal surface tension;
- increasing the strength of the connection.
Most often, a borax solution is used for these purposes, which is prepared from powder and water by heating. Ready-to-use fluxes based on potassium fluoride are also available for sale. If soldering work requires especially careful filling of all surface microdefects, a flux based on potassium tetrafluoroborate will come to the rescue. For reference use only.
GOST 19738-74
By Resolution of the State Committee of Standards of the Council of Ministers of the USSR dated April 26, 1974 No. 1015, the introduction date was set to January 1, 1975
The validity period was removed by Gosstandart Decree dated January 31, 1985 No. 241
1. This standard applies to general-purpose silver solders and establishes grades of solders.
OKP codes for silver solder grades are given in Appendix 3 (Changed edition, Amendment No. 1).
2. The grades and chemical composition of silver solders must correspond to those indicated in the table.
3. The approximate purpose of silver solders is indicated in Appendix 1
4. Data on melting points, density and electrical resistivity of silver solders are given in Appendix 2.
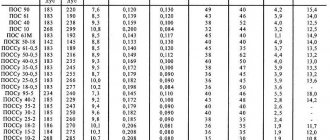
| Solder grade | Chemical composition, % | |||||||||||||
| Silver | Copper | Zinc | Tin | Manganese | Antimony | Phosphorus | Cadmium | Nickel | Lead | No more impurities | ||||
| Gland | Amount of determined impurities | |||||||||||||
| PSR 72 | 72+0,5 | Rest | — | — | — | — | — | — | — | — | 0,005 | 0,10 | 0,005 | 0,10 |
| PSR 71 | 71+0,5 | Rest | — | — | — | — | 1,0+0,2 | — | — | — | 0,005 | 0,15 | 0,005 | 0,15 |
| PSR 70 | 70+0,5 | 26,0+0,5 | Rest | — | — | — | — | — | — | — | 0,050 | 0,10 | 0,005 | 0,15 |
| PSrMO 68-27-5 | 68+0,5 | Rest | — | 5,0+0,5 | — | — | — | — | — | — | 0,005 | 0,15 | 0,005 | 0,15 |
| PSR 65 | 65+0,5 | 20,0+0,5 | Rest | — | — | — | — | — | — | — | 0,100 | 0,10 | 0,005 | 0,15 |
| PSR 62 | 62+0,5 | 28,0+1,0 | — | Rest | — | — | — | — | — | — | 0,005 | 0,15 | 0,005 | 0,15 |
| PSR 50 | 50,0+0,5 | Rest | — | — | — | — | — | — | — | — | 0,005 | 0,15 | 0,005 | 0,15 |
| PSR 50 Kd | 50,0+0,5 | 16,0+1,0 | 16,0+1,0 | — | — | — | — | Rest | — | — | 0,100 | 0,10 | 0,005 | 0,15 |
| PSrKdM 50-34-16 | 50,0+0,5 | Rest | — | — | — | — | — | 31,0+1,0 | — | — | 0,05 | 0,15 | 0,005 | 0,15 |
| PSR 45 | 45,0+0,5 | 30,0+0,5 | Rest | — | — | — | — | — | — | — | 0,050 | 0,10 | 0,005 | 0,15 |
| PSrMCKd 45-15-16-24 | 45+0,5 | Rest | 16,0+1,0 | — | — | — | — | 24,0+1,0 | — | — | 0,150 | 0,15 | 0,005 | 0,15 |
| PSR 40 | 40,0+1,0 | 16,7+0,7 | 17,0+0,8 | — | — | — | — | Rest | 0,3+0,2 | — | 0,050 | 0,10 | 0,005 | 0,15 |
| PSr 37.5 | 37,5+0,3 | Rest | 5,5+0,5 | — | 8,2+0,3 | — | — | — | — | — | 0,050 | 0,10 | 0,005 | 0,15 |
| PSR 25 | 25,0+0,3 | 40,0+1,0 | Rest | — | — | — | — | — | — | — | 0,050 | 0,10 | 0,005 | 0,15 |
| PSR 25F | 25,+0,3 | Rest | — | — | — | — | 5,0+0,5 | — | — | — | 0,010 | 0,15 | 0,010 | 0,15 |
| PSR 15 | 15,0+0,5 | Rest | — | — | — | — | 4,8+0,3 | — | — | — | 0,100 | 0,05 | 0,010 | 0,15 |
| PSR 12M | 12,0+0,3 | 52,0+1,0 | Rest | — | — | — | — | — | — | — | 0,050 | 0,10 | 0,05 | 0,15 |
| PSR 10 | 10,0+0,3 | 53,0+1,0 | Rest | — | — | — | — | — | — | — | 0,050 | 0,10 | 0,05 | 0,15 |
| PSrO 10-90 | 10,0+0,5 | — | — | Rest | — | — | — | — | — | — | 0,200 | 0,15 | 0,010 | 0,30 |
| PSrOSu 8 (VPr-6) | 8,0+0,5 | — | — | Rest | — | 7,5+0,5 | — | — | — | — | 0,200 | 0,20 | 0,015 | 0,40 |
| PSrMO 5(VPr-9) | 5,0+0,5 | 2,0+0,5 | — | Same | — | 1,0+0,2 | — | — | — | — | 0,200 | 0,20 | 0,015 | 0,40 |
| PSrOS 3.5-95 | 3,5+0,4 | — | — | « | — | — | — | — | — | 1,0+0,3 | — | 0,15 | 0,010 | 0,15 |
| PSR 3 | 3,0+0,3 | — | — | — | — | — | — | — | — | Rest | — | 0,15 | 0,010 | 0,15 |
| PSrO 3-97 | 3,0+0,3 | — | — | Rest | — | — | — | — | — | — | 0,200 | 0,15 | 0,010 | 0,30 |
| PSrOS 3-58 | 3,0+0,4 | — | — | 57,8+1,0 | — | 0,5+0,3 | — | — | — | Rest | — | 0,15 | 0,010 | 0,15 |
| PSR 3Kd | 3,0+0,5 | — | 1,0+0,5 | — | — | — | — | Rest | — | — | 0,200 | 0,10 | 0,010 | 0,30 |
| PSR 2.5 | 2,5+0,3 | — | — | 5,5+0,5 | — | — | — | — | — | Rest | — | 0,15 | 0,010 | 0,15 |
| PSR 2.5S | 2,5+0,2 | — | — | — | — | — | — | — | — | Same | — | 0,15 | 0,010 | 0,15 |
| PSR 2 | 2,0+0,3 | — | — | 30,0+1,0 | — | — | — | 5,0+0,5 | — | « | — | 0,15 | 0,010 | 0,15 |
| PSrOS 2-58 | 2+0,3 | — | — | 58,8+1,0 | — | 0,5+0,3 | — | — | — | « | — | 0,15 | 0,010 | 0,15 |
| PSR 1.5 | 1,5+0,3 | — | — | 15,0+1,0 | — | — | — | — | — | « | — | 0,15 | 0,010 | 0,15 |
| PSR 1 | 1,0+0,2 | — | — | 35,0+1,0 | — | 0,9+0,4 | — | 2,5+0,5 | — | « | — | 0,15 | 0,010 | 0,15 |
Notes:
1. In the designation of solder brands, the letters mean: P - solder, Sr - silver, Kd - cadmium, C - zinc, Su - antimony, M - copper, F - phosphorus, O - tin, C - lead. The number after the letter indicates the silver content as a percentage.
2. The zinc content in PSr 72 and PSr 50 alloys should be no more than 0.007%.
Annex 1
Recommended
| Solder grade | Application area |
| PSr 72; PSr 71; PSr 62; PSr 50Kd; PSr 50; PSr 40; PSr 37.5; PSr 25; PSr 15; PSr 10; PSR 2.5 | Tinning and soldering of copper, copper-nickel alloys, nickel, kovar, nickel silver, brass and bronze. |
| PSR 72 | Soldering iron-nickel alloy with silver-plated steel parts. |
| PSr 72; PSr 62; PSr 40; PSr 25; PSR 12M | Soldering steel with copper, nickel, copper and copper-nickel alloys. |
| PSr 72; PSR 62 | Soldering copper with nickel-plated tungsten. |
| Soldering titanium and titanium alloys to stainless steel | |
| PSr 37.5 | Soldering of copper and copper alloys with heat-resistant alloys and stainless steels. |
| PSR 40 | Soldering of copper and brass with kovar, nickel, stainless steels and heat-resistant alloys, soldering of lead-tin bronzes. |
| PSrO 10-90; PSrOSu 8; PSrMO 5; PSrOS 3.5-95; PSrO 3-97; PSrOS 3-58; PSrOS 2-58; PSr2; PSr 1.5. | Soldering and tinning of copper, nickel, copper and copper-nickel alloys with silver-plated ceramics, soldering of silver-plated parts. |
| PSr 3; PSr 2; PSR 1.5 | Soldering copper and nickel with glass enamel and ceramics. |
| PSr 72; PSr 70; PSr 65; PSr 45; PSr 25; PSr 15; PSR 2 | Soldering and tinning of jewelry. |
| PSr 71; PSr 25F; PSR 15 | Self-fluxing solder for soldering copper to bronze, copper to copper, bronze to bronze. |
| PSR 3Kd | Soldering of copper, copper alloys and steels over a freshly applied copper galvanic coating of at least 10 microns. |
| PSrMo 68-27-5; PSrKdM 50-34-16; PSrMCKd 45-15-16-24; PSr 3; PSR 2.5 | Soldering and tinning of non-ferrous metals and steels. |
| PSR 1 | Soldering and tinning of silver parts |
Appendix 2
Information
Data on melting point, density and electrical resistivity of silver solders
| Solder grade | Density kg/m3 | Melting point, K(oC) | Electrical resistivity 10-3 Ohm m | |
| Upper critical point | Lower critical point | |||
| PSR 72 | 10000 | 1052 (779) | 1052 (779) | 2,1 |
| PSr71 | 9800 | 1068 (795) | 918 (654) | 4,3 |
| PSr70 | 9800 | 1043 (770) | 988 (715) | 4,1 |
| PSrMO 68-27-5 | 9900 | 1038 (765) | 928 (655) | 14,0 |
| PSR 65 | 9450 | 995 (722) | 968 (695) | 8,6 |
| PSR 62 | 9600 | 996 (723) | 923 (650) | 25,5 |
| PSR 50 | 9300 | 1133 (860) | 1052 (779) | 2,5 |
| PSR 50 Kd | 9250 | 913 (640) | 898 (625) | 7,8 |
| PSrMCKd 45-15-16-24 | 9400 | 888 (615) | 888 (615) | 6,5 |
| PSrKdM 50-34-16 | 9600 | 958 (685) | 903 (630) | 5,8 |
| PSR 45 | 9100 | 1003 (730) | 938 (665) | 10,0 |
| PSR 40 | 9250 | 883 (610) | 863 (590) | 7,0 |
| PSr 37.5 | 8900 | 1083 (810) | 998 (725) | 37,2 |
| PSR 25 | 8700 | 1048 (775) | 1013 (740) | 7,7 |
| PSR 25F | 8300 | 998 (725) | 918 (645) | 18,6 |
| PSR 15 | 8500 | 1083 (810) | 913 (640) | 20,7 |
| PSR 12M | 8300 | 1103 (830) | 1066 (793) | 7,4 |
| PSR 10 | 8400 | 1123 (850) | 1095 (822) | 7,1 |
| PSrO 10-90 | 7600 | 553 (280) | 494 (221) | 12,9 |
| PSrOSu 8 (VPR-6) | 7400 | 523 (250) | 508 (235) | 19,7 |
| PSrMO 5(VPR-9) | 7400 | 513 (240) | 488 (215) | 15,3 |
| PSrOS 3.5-95 | 7400 | 497 (224) | 493 (220) | 12,3 |
| PSR 3 | 11400 | 588 (315) | 577 (304) | 20,4 |
| PSR 3-97 | 7400 | 498 (225) | 494 (221) | 12,5 |
| PSrOS 3-58 | 8600 | 463 (190) | 453 (180) | 14,5 |
| PSR 3Kd | 8700 | 615 (342) | 587 (314) | 8,0 |
| PSR 2.5 | 11000 | 573 (300) | 568 (295) | 21,4 |
| PSR 2.5S | 11300 | 579 (306) | 577 (304) | 20,7 |
| PSR 2 | 9500 | 511 (238) | 508 (235) | 16,7 |
| PSrOS 2-58 | 8500 | 456 (183) | 456 (183) | 14,1 |
| PSR 1.5 | 10400 | 553 (280) | 546 (273) | 19,1 |
| PSR 1 | 9400 | 508 (235) | 498 (225) | 26,0 |
Appendix 3
Information
| Solder grade | OKP code | Solder grade | OKP code |
| PSR 72 | 17 5232 0006 | PSR 12M | 17 5232 0004 |
| PSR 71 | 17 5232 0007 | PSR 10 | 17 5232 0005 |
| PSR 70 | 17 5232 0001 | PSrO 10-90 | 17 5232 0020 |
| PSrMO 68-27-5 | 17 5232 0008 | PSrOSu 8 (VPR-6) | 17 5232 0021 |
| PSR 65 | 17 5232 0002 | PSrMO 5(VPR-9) | 17 5232 0022 |
| PSR 62 | 17 5232 0010 | PSrOS 3.5-95 | 17 5232 0023 |
| PSR 50 | 17 5232 0011 | PSR 3 | |
| PSR 50 Kd | 17 5232 0012 | PSR 3-97 | 17 5232 0024 |
| PSrKdM 50-34-16 | 17 5232 0013 | PSrOS 3-58 | 17 5232 0025 |
| PSR 45 | 17 5232 0014 | PSR 3Kd | 17 5232 0009 |
| PSrMCKd 45-15-16-24 | 17 5232 0015 | PSR 2.5 | 17 5232 0026 |
| PSR 40 | 17 5232 0016 | PSR 2.5S | 17 5232 0027 |
| PSr 37.5 | 17 5232 0017 | PSR 2 | 17 5232 0028 |
| PSR 25 | 17 5232 0003 | PSrOS 2-58 | 17 5232 0029 |
| PSR 25F | 175232 0018 | PSR 1.5 | 17 5232 0030 |
| PSR 15 | 17 5232 0019 | PSR 1 | 17 5232 0031 |
What fluxes are suitable?
To create a strong connection, it is necessary to properly prepare the surfaces for soldering. To do this, they are cleaned mechanically. Silver solder is used with soldering flux - a liquid or paste that destroys the oxide film and facilitates soldering, increasing the fluidity of the melt.
Borax powder is widely used. It is diluted in a small amount of water, heated and stirred until solder paste is formed.
With this composition you can solder from 490 to 910°C
Ready-made soldering fluids and pastes are also widely available on the market. It is better for a novice master to use purchased materials. Making your own fluxes requires skills in handling strong chemicals.
Soldering process
If you have the appropriate tools, you can carry out soldering yourself. The most difficulties arise when working with stainless steel. The recommendations are as follows:
- To begin with, the surface is cleaned from various contaminants; any mechanical method can be used. Most often, paint and dirt are removed with a brush, after which the surface is degreased using a special composition.
- Selected flux is laid out on the future soldering area. Application technology largely depends on what material is used. The flux must be distributed evenly, otherwise the quality of the connection may be poor.
- To process a large area, a special torch is used to heat the metal to the required temperature. The first sign that the material is ready for soldering is a change in its temperature.
- After reaching the required state of the workpiece, the selected solder is supplied. It should be spread over the surface in an even layer.
- The entire seam is passed from start to finish. A little time is given for the material to cool, after which the workpiece is heated a little more to gradually reduce the temperature.
If the connection area is small, then you can use a small soldering iron. In this case, there is no need to preheat the base.
Technology for creating at home
Preparing solder for soldering silver with your own hands allows you to save significant amounts, but requires melting and foundry equipment and certain skills.
You can do it in the following sequence:
- chop scrap with metal scissors;
- grind the filler material;
- remove iron dust from them with a powerful magnet;
- use precise scales to weigh out 20 gram portions of the charge;
- add 10% borax and mix thoroughly;
- put the weighed portion into a melting spoon and heat with a burner until completely melted;
- warm up the casting mold, called “ingus”;
- pour a spoonful of melt into the ingus in one go;
- cool the mold under running water;
- knock the finished solder out of the mold.
During work, you should be careful, use personal protective equipment, a hood, and do not leave the burner unattended.
Soldering tool
Almost anyone may need to solder a piece of silver jewelry, such as a silver ring with a crack or a broken chain.
Of course, you can turn to a professional jeweler, but such services can be quite expensive.
It is much more economical to do the soldering yourself. This is quite a delicate job, but with due care and minimal soldering skills, it is quite possible to complete it.
Usually in such cases they use an electric soldering iron or a gas torch. The quality of silver soldering will depend on the temperature that the working tool can provide.
It is interesting that not all electric soldering irons provide a melting temperature sufficient for soldering with silver solders.
For example, if an electric soldering iron is designed for solders with a melting point of up to +350 degrees, then it will not be suitable; a much higher temperature is needed here.
In addition, to solder jewelry made of silver of a particular standard, jewelers use special electric soldering irons with thin tips (although standard-sized tips are also suitable for one-time soldering).
An ancient cooking recipe
The recipe includes a Soviet silver fifty-kopeck piece minted in 1924 and a copper nickel minted in 1961.
The sequence of actions is as follows:
- shred coins with scissors;
- melt silver in a spoon;
- add copper to the melting spoon;
- Using smooth circular movements of your hand, roll the melt over the spoon until completely mixed;
- pour the melt into the ingus and cool.
The solder used for soldering silver will be approximately 900 grade. The exact fineness depends on the degree of wear of the coins.